Chlorate is an anion composed of chlorine and oxygen, with a negative charge of -1. The difference in electronegativity between Chlorine and Oxygen is 0.38, making the bond between them non-polar in nature. The chlorate ion has eight different resonance structures, each with octets on all atoms and formal charges of -1 on all oxygen atoms and +2 on the chlorine atom.
Due to its negative charge, chlorate can form compounds with oher elements and ions. These compounds are referred to as chlorates and are often used in industrial applications such as bleaches, detergents, matches, gunpowder and rocket fuel. Chlorates can also be used in agriculture as a fertilizer or herbicide.
The most common chlorate is sodium chlorate which is produced by electrolysis of sodium chloride (table salt) solution. It is widely used in industries such as paper manufacturing, metalworking and water treatment as it has strong oxidizing properties which make it useful for removing impurities from wastewater. It can also be used to produce chlorine dioxide which is a powerful bleaching agent used in paper mills and textile mills.
In conclusion, the chlorate ion carries a negative electrical charge due to its difference in electronegativity between chlorine and oxygen atoms. This makes it useful for many industrial applications such as bleaches, detergents, matches, gunpowder, rocket fuel and water treatment processes.
The Negative Charge of ClO3
The ClO3 molecule has a negative charge because the chlorine atom has a higher electronegativity than the oxygen atoms. This means that the electron distribution within the molecule is asymmetrical, with more electrons being attracted to the chlorine atom than the oxygen atoms. This results in a net negative charge of -1 on the molecule, making it an anion (a negatively charged ion).
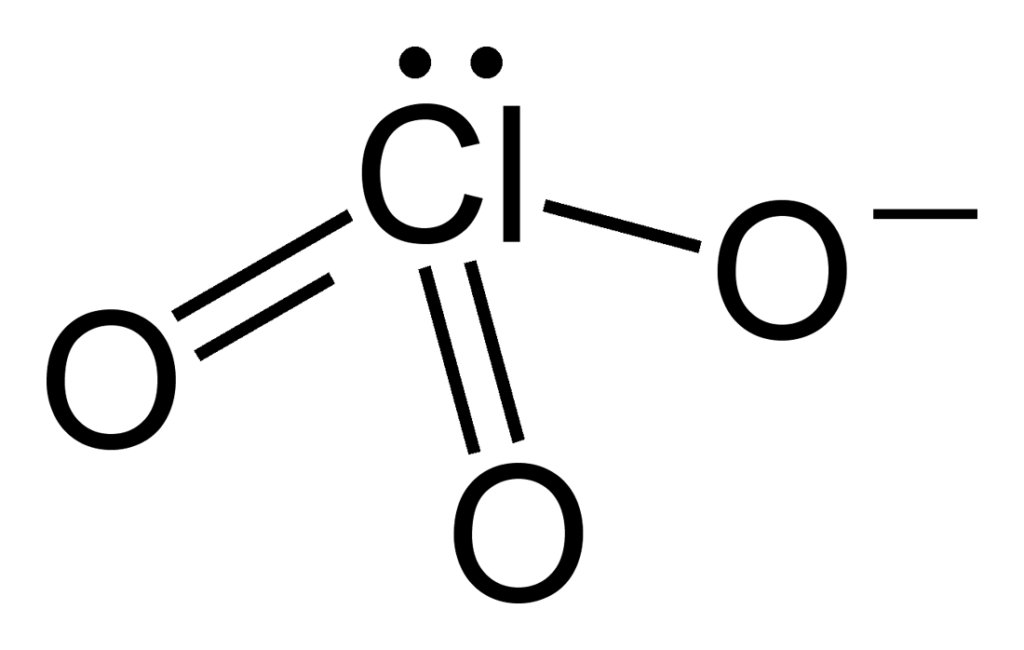
Does Chlorate Have a Positive Charge?
No, the chlorate ion does not have a 2 charge. The total charge of the chlorate ion is -1, as each oxygen atom has a -1 formal charge and the chlorine atom has a +2 formal charge. This can be seen in one of the resonance structures of the chlorate ion (Structure 1), which shows octets on all atoms and formal charges of -1 on all O atoms and +2 on the Cl atom.
The Charge of Chlorate
Yes, chlorate is a negatively-charged anion. It contains one chlorine atom and three oxygen atoms bound together, with a charge of -1. Chlorate is one of the many types of anions that contain chlorine, and all are negatively charged due to their extra electron.
The Charge of Chlorate
Chlorate has a 1 charge because it is an ionic compound formed when chlorine donates an electron to oxygen. The chlorine atom loses one of its 7 valence electrons, leaving it with 6 valence electrons and a formal positive charge of +1. Meanwhile, the oxygen atom gains the electron from chlorine and now has 8 valence electrons, giving it a formal negative charge of -1. The combination of these two ions forms the chlorate molecule with an overall net charge of 1+.
Does ClO3 Have an Electric Charge?
Yes, ClO3 has a charge. The molecular formula of ClO3 is Cl-O-O-O, which indicates that it has an overall negative charge of -1. This is because Cl has an atomic number of 17 and 3 oxygen atoms have an atomic number of 8. The difference in the charges between the two elements gives the molecule a net negative charge of -1.

Is ClO3 a Cation or an Anion?
ClO3 is an anion. It is composed of one chlorine atom and three oxygen atoms, and carries a net charge of -1 due to the number of electrons being greater than the number of protons. Chlorine has an oxidation state of +5 in this molecule, meaning it has gained 5 electrons. This means that ClO3 is a negatively charged ion, or anion.
What Has a Positive Two Charge?
The +2 charge is a common charge for many transition metal ions in the periodic table. These include copper (Cu2+), iron (Fe2+), cobalt (Co2+), nickel (Ni2+), chromium (Cr2+), molybdenum (Mo2+), and tungsten (W2+) among others. Additionally, zinc (Zn2+) and cadmium (Cd2+) also have a +2 charge, although these two elements are not considered transition metals. This charge is also found in some non-metal ions such as sulfate (SO42-) and carbonate (CO32-). The +2 charge is formed when an atom loses two electrons from its outermost shell, leaving it with eight valence electrons.
The Reason for the Negative Charge of Chlorine
Chlorine has 17 protons and 18 electrons, meaning it has 1 more electron than protons. This imbalance of protons and electrons gives chlorine an overall negative charge, because electrons have a negative charge while protons have a positive charge. The net effect of the additional electron is that chlorine has an overall negative charge.
Is Chloride a Negative Ion?
Yes, chloride is a negative ion. It is formed when the element chlorine, a halogen, gains an electron or when a compound like hydrogen chloride is dissolved in water or other polar solvents. The chloride ion has the chemical formula Cl− and carries a single negative charge. This makes it an anion (a negatively charged ion).
Negative Charge
Electrons are negatively charged particles. They have a negative electrical charge, which is opposite to that of the positively charged protons in the nucleus of an atom. Electrons exist in orbitals around the nucleus and can move from one orbital to another. When electrons move from one place to another, they cause a flow of electricity, which is what powers our devices.
The Charge of a +1 Ion
A proton has a positive electrical charge of one (+1). Protons are found in the nucleus of an atom, and they are made up of three quarks. Protons also have a mass of 1 atomic mass unit (amu), which is approximately 1.67×10−27 kilograms. When two or more protons interact with each other, they create an electrostatic force that holds them together to form atoms. This is why protons are often considered the “building blocks of matter”.
The Nature of a Chlorate Ion
The chlorate(I) ion, ClO–, is an oxidizing agent composed of one chlorine atom and one oxygen atom. The chlorine atom in the chlorate(I) ion has an oxidation state of +1, meaning that it has lost one electron. The ion is a strong oxidizer that can be used to disinfect water or bleach fabrics. Chlorate(I) ions can also be used as a pesticide to control certain types of insects.
Does Group 1 Have a Positive Charge?
Yes, Group 1 elements form 1+ ions. This means that Group 1 elements have a +1 charge when they are part of an ionic compound. The atoms of Group 1 elements are always missing one electron from their outermost shell, which gives them a net positive charge and makes them cations with a +1 charge. Examples of Group 1 elements include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs) and francium (Fr).
Conclusion
In conclusion, the chlorate ion has a negative charge of -1 due to its resonance structures. The difference in electronegativity between chlorine and oxygen is 0.38, indicating that the bonds between them are non-polar. As a result, the chlorine atom has 7 valence electrons and is formally neutral while the oxygen atom has 7 valence electrons and carries a formal negative charge. This structure results in an overall negative net dipole moment for the chlorate ion.
