Welcome to the blog post about the charge of SO4! Sulfuric acid (H2SO4) is a strong acid that dissociates in water to form sulfate (SO4-) and hydronium (H3O+) ions. The sulfate ion carries a charge of -2, meaning it has two electrons more than it needs for a neutral state. This negative charge is due to the presence of four oxygen atoms surrounding a single sulfur atom in the molecular structure.
The sulfur atom has six valence electrons which form three bonds with three of the oxygen atoms, creating two single bonds and one double bond. These three bonds are covalent in nature, meaning they share electrons to form a stable molecule. The remaining oxygen atom has an unshared pair of electrons that creates an ionic bond with the sulfur atom, forming a negative charge on the sulfate ion.
The oerall charge of SO4- can be determined by taking into account all the bonding and lone pairs of electrons present in the molecule. For example, each oxygen atom contributes 6 valence electrons to SO4-, totaling 24 valence electrons for four oxygen atoms. Since sulfur only has 6 valence electrons, there must be 2 extra electrons for a total of 30 valence electrons present in SO4-. When subtracting this from 32 total needed for a neutral state, this gives us -2 charge on the sulfate ion.
We hope you found this blog post informative about understanding the charge on Sulfuric acid’s anion, SO4-. Understanding and recognizing charges like this can be helpful when analyzing chemical reactions or when predicting products from certain equations. Thanks for reading!
What Gives SO4 a Charge of 2?
The sulfate anion (SO4 2-) has a charge of -2 because it contains four oxygen atoms bonded to a single sulfur atom. Two of the oxygen atoms are bonded to the sulfur by double bonds (S=O), and the other two oxygen atoms are bonded to the sulfur by single bonds (S-O). These four bonds all have an electron deficiency, resulting in an overall negative charge of -2 on the sulfate anion.
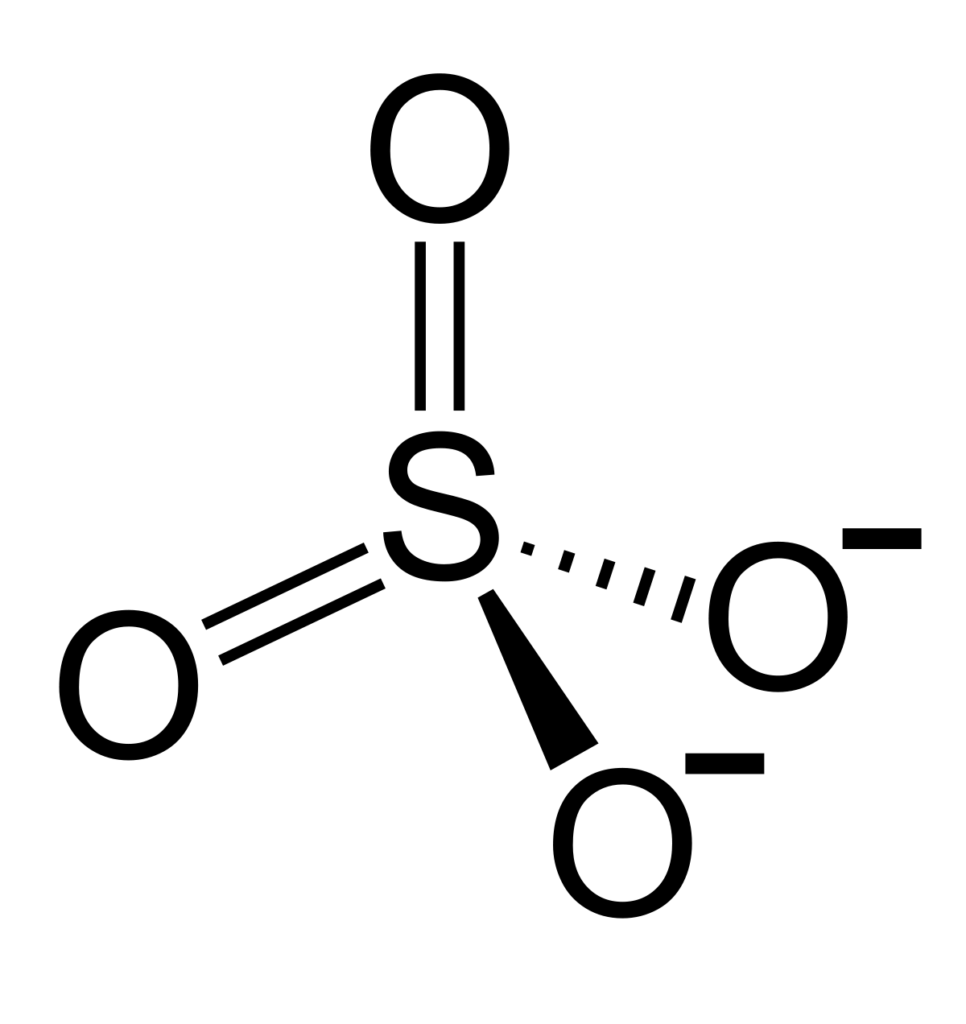
Charge of Sulfate (SO4)
Sulfate, or SO4, is a polyatomic ion with 1 sulfur atom and 4 oxygen atoms. It has a total of 6 sulfur valence electrons and 24 oxygen valence electrons, giving it a total charge of -2.
Ion of SO42
The ion of SO42 is a polyatomic anion, meaning it is composed of multiple atoms bound together. The empirical formula for this anion is SO2−4, with two sulfur atoms and four oxygen atoms forming the anion. This ion is commonly known as the sulfate or sulphate ion and can be found in many different compounds. Salts, acid derivatives, and peroxides containing sulfate are widely used in industry and can be found in many everyday items from beverages to cosmetics. Sulfates are salts of sulfuric acid and are often prepared from that acid.
Is Sulfate (SO4 2-) an Anion or Cation?
The SO4 2 ion is an anion. It consists of one sulfur atom and four oxygen atoms, with the overall charge being -2. This makes it a polyatomic anion, which is a negatively charged ion composed of two or more atoms.
What is the Number of SO4?
The number of SO4 is 4. This is because the chemical formula for sulfate ion (SO42-) tells us that there are four atoms of oxygen and one atom of sulfur. Since each oxygen atom has an oxidation number of -2 and the sulfur atom has an oxidation number of +6, when you add the oxidation numbers together, you get a total of 4.
Source: nature.com
Conclusion
In conclusion, the sulfate ion (SO4) has a charge of -2. This is because it contains 1 sulfur atom with 6 valence electrons and 4 oxygen atoms with 24 valence electrons, resulting in a -2 charge. Two of the oxygen atoms form S=O bonds and the other two form S-O- bonds, creating an anion that carries the negative charge.
