Fluorine is an important element found in nature, and it’s used in many industrial applications. Its charge is a key factor to understand when discussing its properties and behavior. In this blog post, we’ll take a closer look at the charge of fluorine and how it affects the element’s behavior.
Fluorine is the ninth element on the periodic table, with an atomic number of nine (9). This means it has nine electrons in its outer shell. Since it is a non-metal, fluorine wants to gain one electron to form an ion with a -1 charge. When this happens, it becomes an anion. Anions are negatively charged ions that have one or more extra electrons than protons.
The -1 charge of fluorine plays a key role in its behavior when combined with other elements or compounds. It has a high electronegativity because its electron cloud repels other electron clouds away from itself. This high electronegativity allows fluorine to form strong bonds with other atoms or molecules, making it useful for many industrial applications such as refrigerants and plastics manufacturing.
It’s also important to note that the number of electrons in fluorine is equal to its atomic number minus its charge. In this case, nine (9) electrons are equal to nine (9) minus the -1 charge of fluoride, which equals eight (8). This helps us understand why fluoride typically gains one electron when forming ions; it needs to complete its octet so that all eight valence electrons are paired off and stabilized wihin their respective shells.
In conclusion, understanding the charge of fluorine is essential for understanding how it behaves when combined with other elements or compounds. Its -1 charge allows it to have a high electronegativity and form strong bonds with other atoms or molecules. Additionally, since the number of electrons in fluorine equals its atomic number minus its charge, we can see why fluoride typically gains one electron when forming ions; it needs to complete its octet so that all eight valence electrons are paired off and stabilized within their respective shells.
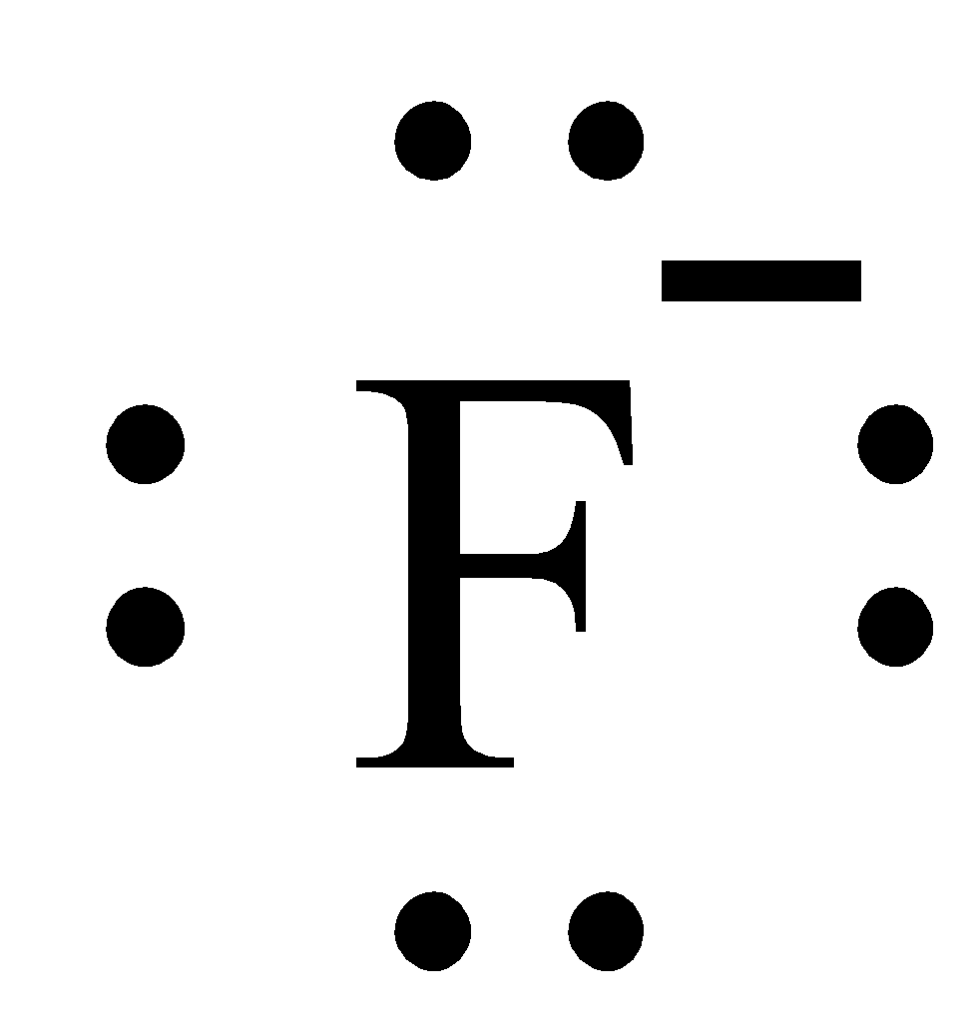
The Charge of Fluorine
Fluorine has seven valence electrons, which is one electron shy of the octet rule. The octet rule states that atoms are most stable when their outermost shell holds eight electrons. To achieve a full octet and become more stable, fluorine will gain one electron to form an ion with a 1- charge. This is why fluorine has a 1 charge – it gains one extra electron to satisfy the octet rule and become more stable.
Charge of Fluorine
Fluorine has a negative charge because it has an extra electron. This makes it an anion, which is a negatively charged ion. Anions are atoms that have one or more extra electrons. The opposite of an anion is a cation, which is a positively charged ion that has one or more fewer electrons than the neutral atom.
How Many Electrons Does Fluorine Have?
Fluorine, the ninth element in the periodic table, has a total of 9 electrons in its electron configuration. Its atomic number is 9, and this indicates that it has 9 protons and 9 electrons. Fluorine does not have 10 electrons.
Number of Electrons in Fluorine with a +1 Charge
Fluorine with a +1 charge would have ten (10) electrons. This is because the number of electrons in an atom is equal to its atomic number, which for fluorine is nine (9), plus the charge of the atom. The atomic number of fluorine is nine (9), and when you add its +1 charge, that gives a total of ten (10) electrons.
Can Fluorine Have a Positive Two Charge?
No, fluorine cannot have a positive two charge. Fluorine is an element in the halogen group of the periodic table, and it has seven valence electrons. This means that it can form compounds with either a negative one charge or a neutral charge, but not a positive two charge. Fluorine needs to gain one electron to achieve a +2 charge, which is not possible due to its configuration of electrons.
Can Fluorine Have a Positive Oxidation State?
Yes, fluorine can have a +1 oxidation state. This is due to its high electronegativity, which allows it to form strong bonds with other elements and take on a positive charge. Fluorine is the most electronegative element in the periodic table, and its ability to form strong covalent bonds makes it a good candidate for forming positive oxidation states. For example, when combined with oxygen, fluorine will take on an oxidation state of +1 and form the compound, hydrogen fluoride (HF). In addition to oxygen, fluorine can also form other compounds with elements like chlorine and bromine that may also cotain a +1 oxidation state.
Does Fluorine Have a Negative Charge?
Yes, fluorine is always found to have a negative oxidation state. This is because it is the most electronegative element in the periodic table, meaning that it has an affinity for electrons and tends to attract them from other atoms. As a result, when fluorine is part of a molecule or compound, it will be assigned a negative charge as it has taken electrons away from other atoms in the molecule.
The Significance of Fluorine’s Negative Charge
Fluorine is the most electronegative element due to its atomic structure. It has five electrons in its 2P orbital, which is just one electron short of reaching the optimal electron configuration of six electrons. This makes the electrons tightly bound to the nucleus, giving it a higher negative charge than other elements. The strong negative charge of fluorine allows it to form strong bonds with other atoms or ions, making it an essential component of many chemical reactions.
The Charge of Fluoride Ion
Yes, fluoride ion is a negatively charged ion. It is the simplest inorganic, monatomic anion of fluorine and has an atomic number of 9. The chemical formula for fluoride ion is F−, indicating its negative charge. It is considered a trace element, found in various minerals but only present in trace amounts in water.
Charge of Fluorine 9
The charge of a fluorine atom is neutral, meaning it has the same number of protons (nine) as electrons (also nine). This leaves the atom with no overall electric charge. If a fluorine atom gains an extra electron, it becomes a fluoride ion with a charge of -1.
Is Fluorine Element Number 9?
No, fluorine is not a 9. Fluorine is a chemical element, symbol F and atomic number 9 on the periodic table. It has an atomic weight of 19 and is a gas at room temperature. Fluorine is the most electronegative element in its group, making it very reactive.

Atomic Charge of +1
The proton is a subatomic particle found in the nucleus of an atom, and it has a charge of +1. It has a mass of 1 atomic mass unit (amu), which is equivalent to 1.67 × 10^-27 kilograms. Protons are fundamental particles that form the building blocks of matter, and they are responsible for the electrical attraction between atoms. In a neutral atom, the number of protons must equal the number of electrons in order for the atom to remain electrically neutral. As such, protons can be seen as a crucial ingredient in understanding how atoms are formed and interact with each other.
What Causes an Atom to Have a Positive Charge?
An atom has a +1 charge when it has one more proton than electron. This is because protons are positively charged, while electrons are negatively charged. When an atom has an unequal number of protons and electrons, the net charge is determined by subtracting the number of electrons from the number of protons. If the result is positive, then the atom has a +1 charge. For example, a sodium atom with 11 protons and 10 electrons would have a +1 charge because 11-10=+1.
Charge of an Electron
The charge of an electron is -1. This is a fundamental physical constant that is expressed as 1.602 × 10-19 coulombs. It can also be denoted by the symbol e. Electrons have a negative electric charge, while protons, which are found in the nucleus of an atom, have a positive electric charge. The magnitude of the electric charge of an electron is equal to the magnitude of the electric charge of a proton, but with opposite sign.
Conclusion
In conclusion, Fluorine has a charge of -1 due to the fact that it has one extra electron than the number of protons in its nucleus. This makes it an anion, and the charge is equal to the atomic number minus the number of electrons. Fluorine’s atomic number is 9, and it has 9 electrons in its ground state, thus resulting in a negative charge of -1.
