Have you ever wondered what the charge of ammonium is? Ammonium is an important component of many substances, and understanding its charge can be quite helpful. In this blog post, we’ll take a look at the charge of ammonium and why it matters.
Ammonium is a polyatomic ion with the chemical formula NH4+. It’s composed of one nitrogen atom and four hydrogen atoms, all bonded together in a cationic form. The overall charge of the ammonium ion is +1. This means that when it comes into contact with other compounds, it will attract electrons and form a positive charge.
The major factor that determines the proportion of ammonia or ammonium in water is water pH. Generally speaking, when the pH level of water increases, so does its capacity to hold onto ammonium ions. As temperature increases, however, these ions become more mobile and less likely to remain in solution. Additionally, ionic strength also influences how much ammonium remains in water; higher ionic strength leads to lower concentrations of ammonium.
So why does all this matter? Well, understanding the charge of ammonium can be extremely useful for any number of reasons. For instance, in wastewater treatment plants or sewage systems, knowing how much ammonium is present can help determine how effective certain treatments will be. Additionally, understanding the charge of this compound can also aid in designing more efficient fertilizers for agricultural use.
In conclusion, although the charge of ammonium may seem like an insignificant detail at fist glance, it actually plays an important role in many different situations. Knowing how this compound behaves under different conditions can help us better understand our environment and make more informed decisions about how to approach certain problems.
Does Ammonium Have a Positive Charge?
Yes, ammonium has a +1 charge. The ammonium ion is composed of one nitrogen atom and four hydrogen atoms, and it gains one extra proton in its nucleus compared to a neutral nitrogen atom. This causes it to have an overall positive charge of +1 due to the unequal number of protons and electrons in its nucleus. This is the sole difference between ammonium and a neutral nitrogen atom, as all other characteristics (number of neutrons, electron shells, etc.) remain unchanged.
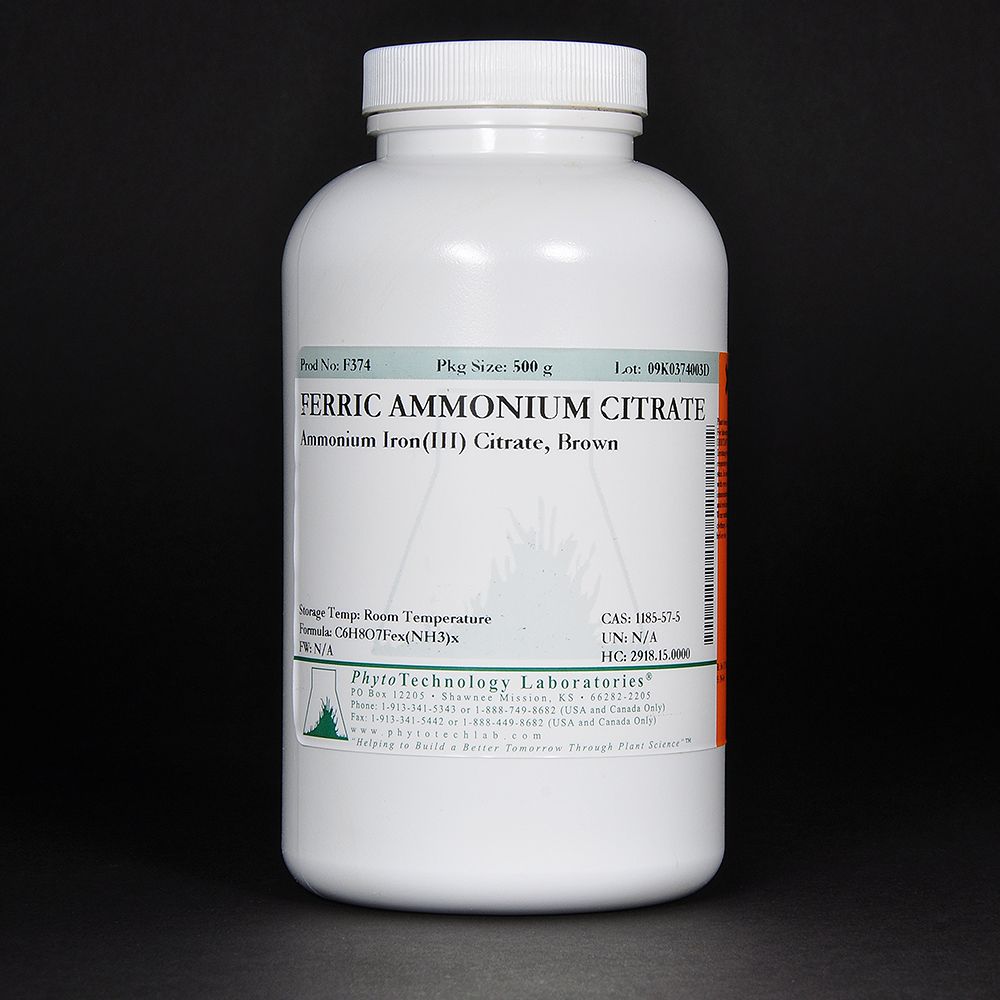
Is Ammonium NH4+?
Yes, ammonium is an ionized form of ammonia, and has the chemical formula NH4+. Ammonia (NH3) is a gas that is found in nature, while ammonium (NH4+) is the ionized form of ammonia, which dissolves in water. At neutral pH levels, ammonia and ammonium are present in equal amounts. However, as the pH level increases or decreases from this neutral point, the relative concentration of each changes. At lower pH levels, more ammonium will be present in water than ammonia, while at higher pH levels, more ammonia will be present than ammonium.
The Charge of NH4
The charge of NH4 is positive, or cationic, because it contains four hydrogen atoms bonded to one nitrogen atom. The nitrogen atom has an atomic number of seven, which means that it has seven protons and seven electrons in its nucleus. Since there are only four electrons shared between the hydrogen atoms and the nitrogen atom, the molecule does not have enough electrons to completely fill its outer valence shell. This leaves the molecule with a net positive charge, due to the excess of protons compared to electrons. The overall positive charge is known as an ammonium ion (NH4+).
Charge of Ammonium
The ammonium cation is a positively-charged ion, meaning it carries a positive electrical charge. Its chemical formula is NH+4 or [NH4]+, which indicates that each ammonium ion contains one positively-charged nitrogen atom and four positively-charged hydrogen atoms. Therefore, the overall charge of an ammonium cation is positive.
Is Ammonium Polar?
No, NH4+1 is not a polar molecule. This is due to its tetrahedral shape, which gives the molecule a symmetrical distribution of charge. The four N-H polar bonds on the molecule have dipole moments that are equal and opposite to each other, thus cancelling each other out and resulting in an overall zero dipole moment for the molecule. Therefore, NH4+1 is a non-polar molecule.
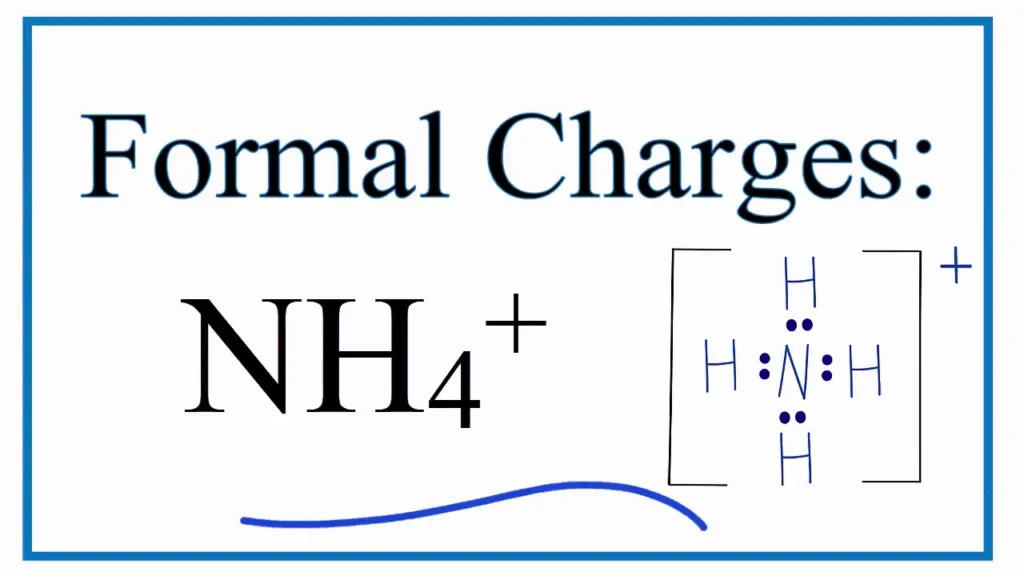
Is Ammonium Positively Charged?
Yes, NH4 is positively charged due to its cationic nature. The NH4 ion has an incomplete octet of electrons, making it electron deficient and giving it a positive charge. This charge is balanced by four hydrogen atoms, forming the complete ammonium ion (NH4+).
Is NH4 Ammonia or Ammonium?
NH4 is ammonium. It is the ionised form of ammonia (NH3). Ammonia is a gas at room temperature, while ammonium is a salt. When ammonia and ammonium are both present in aquarium water they are referred to as Total Ammonia Nitrogen (TAN). NH4 is usually present in greater concentrations than NH3 and is considered to be less toxic to aquatic life.
Is NH3 or NH4+ a Base?
NH3 is the base in the conjugate acid base pair of NH4+ and NH3. NH3 accepts a proton, making it a Bronsted-Lowry base. This contrasts with NH4+, which donates a proton, making it a Bronsted-Lowry acid. Therefore, NH3 is the base and NH4+ is the acid in this pair.
Is Ammonium (NH4) a Positive or Negative Ion?
The ammonium ion (NH4+) is a positively charged ion. It has one more proton than the neutral ammonia (NH3) molecule, resulting in an overall positive charge. The extra proton is balanced by four electrons, which gives the ammonium ion its positive charge.
The Negative Charge of NH4
NH4 is not negatively charged. It has an overall positive charge due to the fact that it has one more proton than electron. NH4 is an ionic compound composed of a nitrogen atom covalently bonded to four hydrogen atoms, forming an ammonium ion (NH4+). The covalent bond between the nitrogen and each hydrogen atom provides each hydrogen atom with a single proton, giving the ammonium ion four protons and three electrons. Since the ammonium ion has one more proton than electrons, it carries a positive charge.
Is the Ammonium Ion Negatively Charged?
Yes, ammonium ion (NH₄⁺) is a negatively-charged ion. This is because the nitrogen atom in ammonium has an extra proton, resulting in an extra electron in its outermost shell of electrons. As a result, it has an overall negative charge due to the presence of more electrons than protons.
The negatively-charged nature of ammonium ion has important implications for acidity, basicity, proton affinity and ion energetics. Ammonium ion is a weak acid when dissolved in water and its dissociation into ammonia and hydronium ions makes the solution slightly acidic. Ammonium also exhibits basic properties due to its ability to accept protons from water molecules and form hydroxide ions (OH⁻).
The high proton affinity of ammonium helps to determine the strength of acids; the higher the proton affinity, the stronger the acid. Additionally, snce ammonium is a charged particle, it has a certain amount of energy associated with it; this energy can be used to drive chemical reactions or interact with other ions.
Conclusion
In conclusion, the ammonium ion is a positively charged polyatomic ion with a charge of +1. It is composed of one nitrogen atom and four hydrogen atoms, with the chemical formula NH4+. The proportion of ammonia or ammonium in water is determined by water pH, as well as temperature and ionic strength. Ammonium has a formal charge of +1, while HN3 has charges of +1 and –1 that balance each other out.
