Carbonyl and carboxyl groups are functional groups that play an important role in chemistry. Carbonyl groups consist of a carbon atom double bonded to an oxygen atom, while carboxyl groups consist of a carbonyl group and a hydroxyl group bonded to each other via the carbon atom of the carbonyl group. The chief chemical characteristic of the carboxylic acids is their acidity.
Carbonyls are important in organic chemistry because they can form several types of bonds with other molecules, including covalent bonds, hydrogen bonds, and ionic bonds. Carbonyls can also act as reducing agents, which means they can donate electrons to other molecules or atoms. In addition, they are found in many compounds such as alcohols, ketones, aldehydes, esters, amides and carboxylic acids. They are also common in proteins as part of their structure and function.
Carboxyl groups differ from carbonyls in that they contain an additional hydroxyl group (-OH) bound to the same carbon atom. Unlike carbonyls, carboxyl groups form only covalent bonds with other molecules or atoms. They are also acidic due to the presence of the -OH group attached to the same carbon atom as the carbonyl group. Carboxylic acids have a generic formula of RCO2H where R is any substituent attached to the carbon-oxygen double bond.
Carbonyls and carboxyls often react differently with other molecules due to their distinct structures and properties. Carbonyls typically undergo nucleophilic additions wereas carboxylic acids do not typically undergo nucleophilic additions but instead lead to acid-base reactions when exposed to bases or acids respectively due to their acidic nature.
Overall, both carbonyl and carboxyl groups are important functional groups found in organic chemistry that have unique properties which make them very useful in various applications such as medicine, drug development and enzyme catalysis among others. It is important for chemists to understand their structures and how they interact with other molecules so that they can use them effectively for different purposes.
Difference Between Carbonyl and Carboxyl
The difference between carbonyl and carboxyl groups lies in the number of attached atoms. The carbonyl group consists of one carbon atom double-bonded to an oxygen atom (C=O), while the carboxyl group consists of a carbonyl group and a hydroxyl group (OH) bonded to the same carbon atom (C–O–H). As a result, the carboxyl group has two oxygen atoms attached to the same carbon atom, compared to just one oxygen atom in the carbonyl group. This relationship gives carboxylic acids their characteristic acidic properties, while carbonyls are generally neutral. In addition, carboxylic acids often form clusters or dimers due to hydrogen bonding between adjacent OH groups in the carboxyl group, whereas this is not pssible for carbonyls.
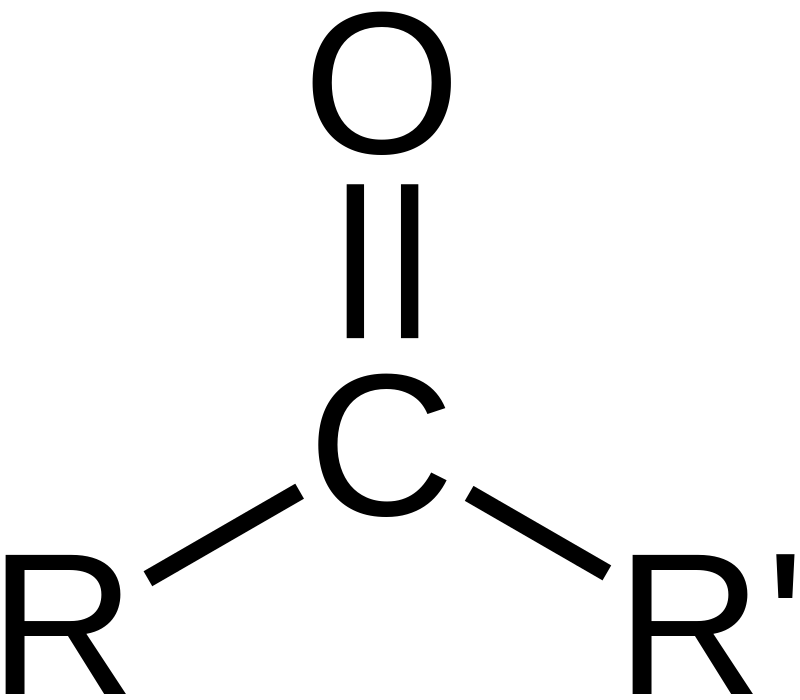
The Difference Between Carbonyl and Carboxyl Groups
The COOH group is a type of carboxyl group, which is composed of a carbonyl group (C=O) and a hydroxyl group (-OH). The carbonyl group provides the acidic properties to the carboxyl group, which makes it an important functional group in organic chemistry. Carboxylic acids are molecules that contain at least one carboxyl group and they are known for their acidic properties. As such, COOH is both carbonyl and carboxyl.
Are Carbonyl Groups Carboxylic Acids?
No, a carbonyl group is not a carboxylic acid. A carbonyl group refers to any functional group with a carbon atom double-bonded to an oxygen atom. A carboxylic acid, on the other hand, is a specific type of organic compound with the general formula RCO2H, where R is an alkyl or aryl group. To be considered a carboxylic acid, one of the substituents on the carbonyl group must be an OH group.
Why COOH Is Not Considered a Carbonyl Group
Carboxylic acids, represented as COOH, are not considered to be a carbonyl group because the lone pairs on oxygen atom attached to hydrogen atom in the −COOH group are involved in resonance and are not available for reaction. This makes the carbon atom less electrophilic and, therefore, carboxylic acids do not exhibit the same properties as oher carbonyl compounds. Additionally, carboxylic acids contain a hydrogen atom attached to the oxygen atom of the C=O double bond which is absent in other true carbonyl compounds. Therefore, this difference in structure prevents carboxylic acids from being classified as a carbonyl group.
Are COO and COOH the Same?
No, COO and COOH are not the same. COO is the molecular formula for a carboxylate anion, which is formed when a carboxylic acid group (COOH) loses its hydrogen (becomes deprotonated). The difference between the two can be seen in their molecular structures: COO has an extra oxygen atom with a negative charge, while COOH does not. The pH of the solution determines whether a carboxylic acid will remain in its protonated form (COOH) or become deprotonated to form a carboxylate anion (COO).
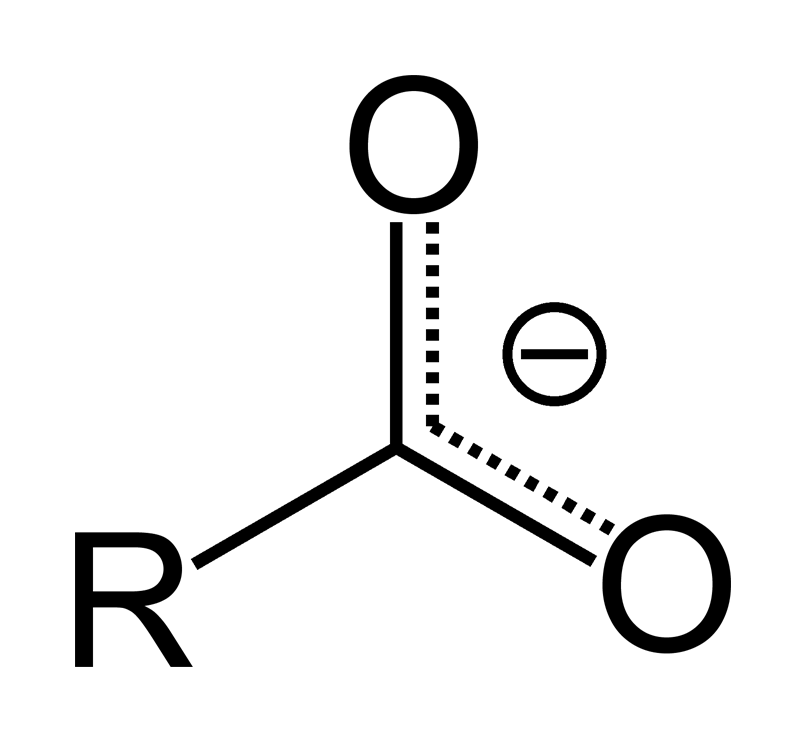
What is the Alternative Name for Carbonyl?
Another name for carbonyl is carbon-oxygen double bond, which refers to the chemical structure of carbonyl compounds, in which a carbon atom is double-bonded to an oxygen atom. This type of bond is referred to as a carbon-oxygen π bond or C=O bond. It is also sometimes referred to as a “carbonyl group,” since the presence of this type of bond indicates that the compound contains a carbonyl functional group.
The Role of the COOH Functional Group
The COOH functional group is a carboxyl group composed of a carbonyl group bound to a hydroxyl group. It is an important functional group found in many molecules throughout nature, including amino acids and fatty acids. The structure of this functional group is often written in condensed form as –CO2H or –COOH and consists of a carbon atom double-bonded to an oxygen atom, with a single-bonded oxygen atom attached to the carbon which has another single-bonded hydrogen atom attached. This particular combination of atoms givs the carboxyl group its acidic properties, making it capable of donating protons to other molecules and acting as an acid catalyst in biochemical reactions.
Difference Between Carboxyl and Carboxylic
The main difference between carboxyl and carboxylic is that carboxyl is a functional group, while carboxylic refers to an entire class of organic compounds cntaining the carboxyl functional group. Carboxyl is composed of a single C=O (carbonyl) bond with a hydroxyl (−OH) group attached to the carbon atom. This is what gives the carboxyl group its characteristic acidic properties. On the other hand, carboxylic acids are organic compounds that contain the carboxyl functional group and have the general formula R−COOH. Carboxylic acids can be further classified into different types based on their chemical structure and properties, such as mono-carboxylic acids, dicarboxylic acids, polycarboxylic acids, etc.
Identifying the Functional Group with COOH
The COOH functional group is a carboxylic acid, which is composed of an alcohol group (hydrogen bound to an oxygen) and a carbonyl group (carbon double bound to an oxygen). Carboxylic acids are commonly abbreviated as COOH in chemical literature.
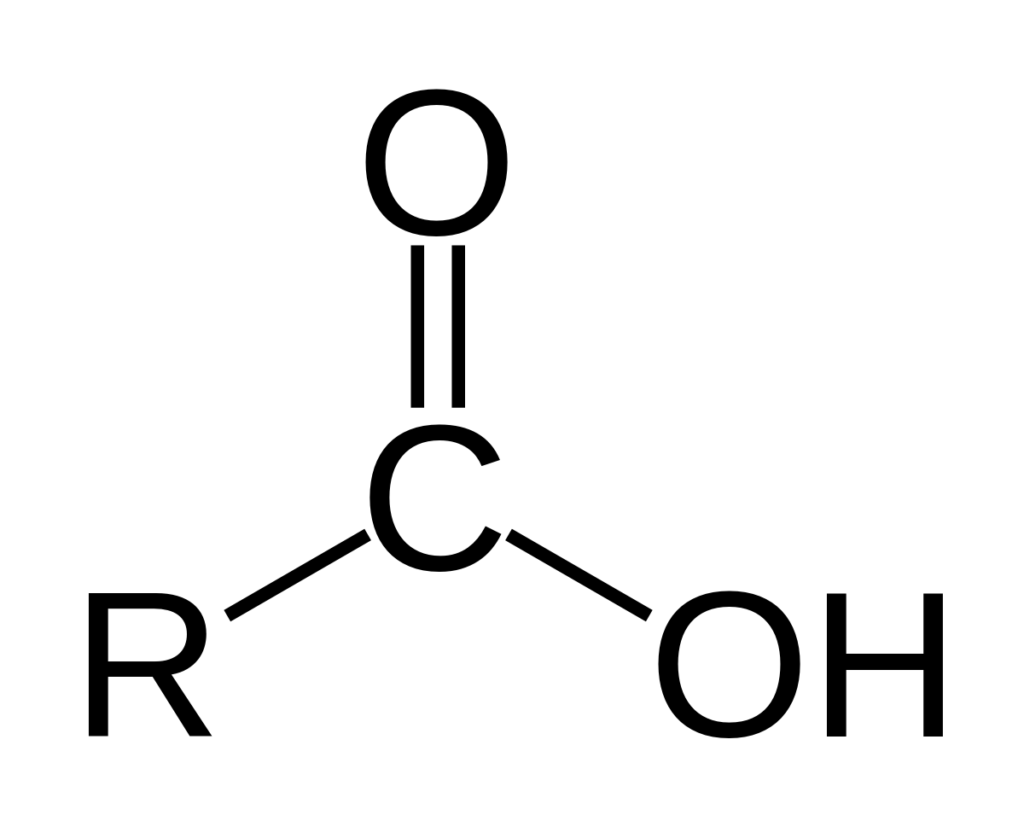
Relationship Between Carboxyl and Carbonyl Groups
The carboxyl group is closely related to the carbonyl group due to thir chemical structure. The carboxyl group is a combination of a carbonyl and hydroxyl group, both of which are bound to a carbon atom. The carbonyl group consists of a carbon double-bonded to an oxygen atom, and the hydroxyl group is an OH bond. When these two groups are combined, they form a carboxyl group which is essential in many biochemical reactions. This combination can be used to catalyze the formation of amides from acids or esters, and can be used as a leaving group in nucleophilic substitution reactions.
The Carbonyl Group
The carbonyl group is a functional group found in organic molecules that consists of a carbon atom double-bonded to an oxygen atom (C=O). This structure is also referred to as a “carbonyl” or an “oxo” group. Its two most common forms are aldehydes and ketones, which are both characterized by the presence of the carbonyl group and an attached carbon-containing compound. Aldehydes are characterized by the presence of a hydrogen atom attached directly to the carbonyl carbon, whie ketones feature two alkyl groups bound to the carbon. Carbonyl groups have important implications for smell and taste in many aromatic compounds.
Types of Carbonyl Groups
The two types of carbonyl groups are aldehydes and ketones. An aldehyde is an organic compound containing a carbonyl group bonded to at least one hydrogen atom. Aldehydes are generally characterized by the presence of a formyl group, whch often has the structure R-CHO, where R represents an alkyl or other organic group. Ketones, on the other hand, are organic compounds with a carbonyl group bonded to two other carbon atoms. Ketones can have the structure R2CO, where R represents an alkyl or other organic group. In general, aldehydes have higher reactivity than ketones due to the presence of the hydrogen atom in the carbonyl group.
Which Functional Group is Not Carbonyl?
A functional group that is not a carbonyl is an ether. Ethers are organic compounds typically containing two alkyl or aryl groups bonded to oxygen. They are polar molecules with low boiling and melting points, making them useful as solvents and intermediates in organic synthesis. Unlike other functional groups, ethers do not contain any atoms with a double bond to an oxygen atom, which means they are not classified as carbonyls.
Absence of Carboxyl Group
Yes, there is no carboxyl group in picric acid. A carboxyl group is a functional group consisting of a carbonyl and a hydroxyl group, bonding to the same carbon atom. Picric acid, on the other hand, has two nitro groups instead of the hydroxyl group, making it an aromatic nitro compound. This difference in structure is what makes picric acid distinct from other carboxylic acids.
Is COOH a Ketone?
No, COOH, or carboxylic acid, is not a ketone. A ketone is an organic compound that contains a carbonyl group bonded to two additional carbon atoms. The carbonyl group in a ketone is usually denoted by the symbol R-CO-R’, where R and R’ can be alkyl or aryl groups. On the other hand, carboxylic acids contain the –COOH functional group, which includes an oxygen atom double-bonded to a carbon atom, along with the hydroxyl (-OH) group attached to the same carbon atom. Therefore, carboxylic acids are not classified as ketones.
Conclusion
In conclusion, carbonyl and carboxyl groups are similar in terms of the fact that they both consist of a carbon atom double-bonded to an oxygen atom. However, a carboxyl group is distinct in that it also contains a hydroxyl group attached to the carbonyl group via the carbon atom, giving it acidic properties. Carboxylic acids do not give the same characteristic reactions of carbonyl groups due to the lone pairs on oxygen atoms being involved in resonance and making the carbon atom less electrophilic.
