Expanded octet is a concept in chemistry that describes molecules and ions which have more than eight electrons arond the central atom. This concept is particularly relevant to elements of the third period and beyond, as these are capable of exceeding the octet rule by having more than eight electrons around the central atom.
Atoms of the second period, such as carbon, nitrogen, oxygen and fluorine cannot expand their octet, meaning they must obey the octet rule and only have eight valence electrons around the central atom. However, atoms of the third period and beyond are able to exceed this limit by forming an expanded octet. This is possible due to their energetically accessible, or low lying d-subshell for bonding. Common examples of elements that form an expanded octet include sulfur, phosphorus, silicon, and chlorine.
Molecules such as phosphorus pentachloride (PCl 5) and sulfur hexafluoride (SF 6) are examples of molecules that deviate from the octet rule by having more than 8 electrons around their central atom. These molecules have a fully expanded octet with eighteen valence electrons surrounding their central atom – ten from the outer shell and eight from its inner shell.
The expanded octet has important implications for chemical reactions since it increases the number of electron pairs available for bonding in large molecules or ions containing multiple atoms. This allows them to form stronger bonds with other atoms or molecules in order to achieve greater stability and reactivity. It also means that these molecules can form multiple bonds with other atoms or molecules without violating any existing rules governing chemical reactions.
In summary, while atoms in Period 2 cannot expand their octets due to a lack of available d-subshells for bonding, atoms in Period 3 and beyond can exceed this limit by forming an expanded octet with more than 8 electrons surrounding its central atom. This makes it possible for larger molecules or ions containing multiple atoms to form stronger bonds with other atoms or molecules in order to achieve greater stability and reactivity!
Can a Valence Shell Be Expanded?
No, N cannot have an expanded valence shell. The valence shell of nitrogen is the second (n = 2) shell and consists of just two subshells: the 2s and 2p. There is no such thing as a 2d orbital, so nitrogen cannot expand its valence shell beyond the 2s and 2p subshells. As a result, nitrogen can form NF3 (in which nitrogen has an octet) but not NF5.
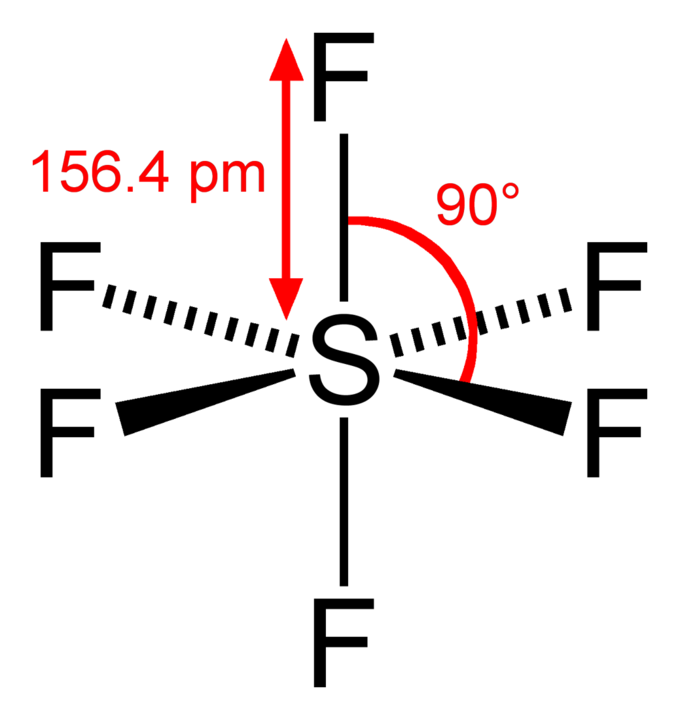
Elements That Cannot Have an Expanded Octet
Elements in Period 2 of the periodic table (C, N, O, and F) cannot have an expanded octet due to the limitations of their energetically accessible d-subshells. This means that they must obey the octet rule, by forming only four bonds with other elements in order to achieve a full outer electron shell. Period 3 and below elements are able to expand their octets by making use of the additional electrons available in their low lying d-subshells.
Can the Octet Rule be Exceeded?
Yes, n can exceed the octet rule. Atoms of the third period and beyond are capable of haing more than eight electrons around the central atom, which exceeds the octet rule. This is possible due to the increased number of orbitals in these periods, which allows for more electrons to be held by the atom. Additionally, atoms with an odd number of electrons can form an expanded octet structure, which also allows for more than eight electrons around the atom. This is known as a “pentad” or “nonet” structure, as five or nine electrons are present around the central atom.
Expanded Octet Elements
An expanded octet is a situation in which more than 8 electrons surround the central atom of a molecule. This type of electron configuration is most often observed in molecules with atoms from the third period and beynd on the periodic table. Elements such as sulfur, phosphorus, silicon, chlorine, boron, nitrogen, oxygen and fluorine can all form an expanded octet. Depending on the molecule’s structure, these elements may gain or lose electrons to achieve the desired electron configuration. For example, sulfur atoms can gain two additional electrons to form sulfur tetrafluoride (SF4) or lose six electrons to form sulfur hexachloride (SCl6). Similarly, chlorine atoms can gain four additional electrons to form phosphorous pentachloride (PCl5) or lose two electrons to form dichlorine hexoxide (Cl2O6).
The Octet Rule for Nitrogen
The octet rule is a concept in chemistry that states that atoms tend to react in such a way so as to achieve a full valence shell of 8 electrons. This is usually accomplished by forming chemical bonds with other atoms, either through sharing electrons or donating or accepting them. In the case of nitrogen (N), it has 5 valence electrons and needs 3 more to complete its octet. This can be accomplished by forming three covalent bonds with other atoms, allowing it to share three electron pairs and have a full octet.
Valence Shell of Nitrogen
The valence shell of Nitrogen (N) is determined by the number of electrons it has in its outermost shell. Nitrogen has two possible configurations, with either three or five valence electrons. When nitrogen has three valence electrons, they are located in the 2s orbital and one 2p orbital. When nitrogen has five valence electrons, they are located in the 2s orbital, two 2p orbitals, and one lone electron. Therefore, the valence shell of Nitrogen can be represented as either 2s2p or 2s2p1 depending on the number of valence electrons it contains.
Exceptions to the Octet Rule
The octet rule states that atoms in a molecule tend to bond together in such a way that each atom has eight electrons in its valence shell. However, there are three major exceptions to this rule. The frst exception is molecules with an odd number of electrons, such as NO, which have nine electrons. The second exception is molecules with one or more atoms possessing more than eight electrons, such as SF6, which has twelve electrons. Finally, the third exception is molecules with one or more atoms possessing less than eight electrons, such as BCl3 which only has seven electrons.
Exceptions to the Octet Rule
1. Lone pairs: When an atom has more than 8 valence electrons, it can form additional lone pairs not included in the octet. This is especially common in molecules containing nitrogen and oxygen, which often form five- or six-member rings.
2. Expanded octets: Atoms such as sulfur, phosphorus, and chlorine may have more than 8 electrons in their valence shell, allowing them to form an expanded octet with additional electron pair bonds.
3. Ions: Ions with less than 8 electrons may still be stable due to the stability of their electronic structure. For example, a single atom of potassium has only one valence electron but is still stable because it forms a filled inner shell of eight electrons.
4. Odd electron molecules: Molecules with an odd number of electrons can break the octet rule by forming three-center four-electron bonds known as hypervalent molecules or biradicals.
5. Hypercoordinated molecules: Large molecules can cotain atoms that are hypercoordinated, meaning they are bonded to more than 8 other atoms at once. These molecules are often stabilized by resonance effects between different Lewis structures that satisfy the octet rule for each individual atom but require more than 8 electrons on a single atom to explain all of the observed bonding patterns in the molecule.
Elements That Do Not Follow the Octet Rule
The three elements that do not follow the octet rule are hydrogen, beryllium, and boron. Hydrogen has only one valence electron, while beryllium and boron each have two. This means that these three elements cannot form an octet of electrons as other atoms typically do. As a result, they tend to form molecules with fewer than eight electrons around their nucleus. This is why these elements are exceptions to the octet rule.
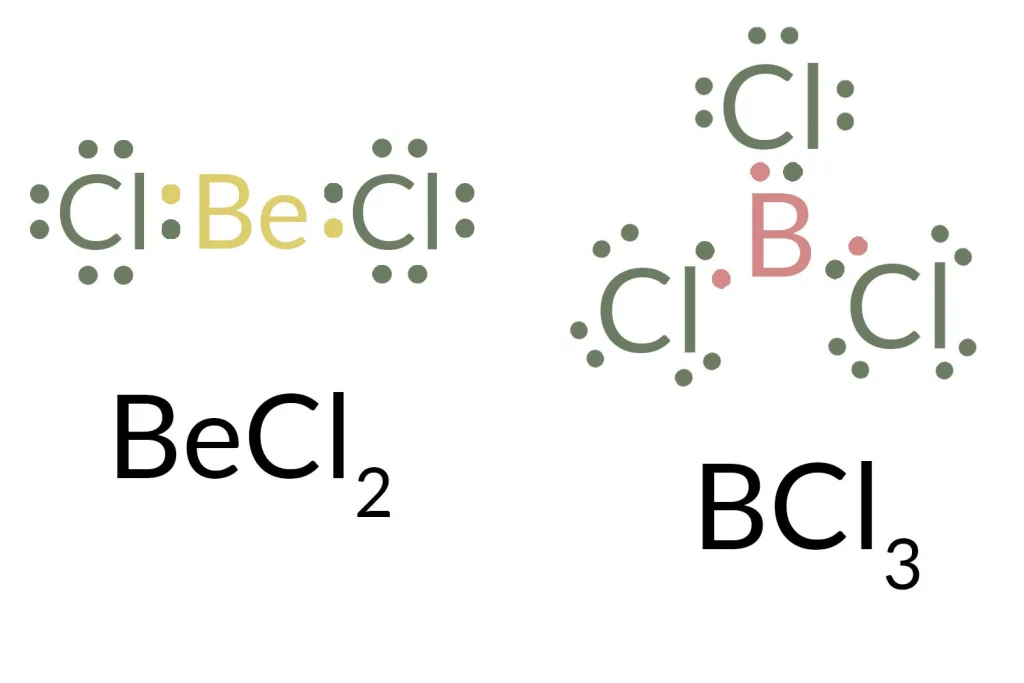
Number of Electrons Needed for N to Fill Its Octet
Nitrogen needs to gain 3 electrons to fill its octet. This is because the nitrogen element has 5 valence electrons in its outermost shell, and it requires 8 electrons in order to complete its octet. When an atom gains or loses electrons, it does so in order to achieve a stable electron configuration that is most energetically favorable for the atom.
Can Hypervalency Exist in Nitrogen?
Yes, n can be hypervalent. Hypervalency occurs when atoms have more than eight electrons in thir valence shell. This is possible for atoms with n≥3 due to the fact that they are able to break the octet rule by having more than eight electrons. This allows them to form five or more bonds, something that is nearly unheard of for atoms with n≤2. Hypervalency can also occur in molecules with a central atom of n≥3, where the octet rule cannot be satisfied by all of the atoms involved in the bonds. In these cases, some of the atoms involved in bonding may have more than eight electrons in their valence shell, leading to hypervalency.
Understanding Expanded Octets
Expanded octets, also known as hypervalent molecules, are molecules with more than the typical 8 valence electrons in their outer-most shell. This is achieved by havng more atoms bonded to the central atom or by having non-bonding electrons present. This is often seen in molecules that contain elements like nitrogen, phosphorus, sulfur, and chlorine. These elements can form bonds with other atoms in order to achieve a full octet of electrons. In some cases, however, these elements will form bonds with multiple different atoms at once and thus end up with an expanded octet of 10 or 12 electrons. This allows for a greater variety of chemical reactions and provides greater stability to the molecule than what would be expected from a standard octet.
Expanded Octet in a Row
The elements in the third row and below can have an expanded octet. This is because these elements are part of the d orbital, which can hold up to ten electrons. Due to this increased capacity, these elements can form more than eight bonds, leading to an expanded octet.
Conclusion
In conclusion, the expanded octet is a concept where atoms from period 3 and below can expand their valence shell beyond 8 electrons. This allows for molecules like PCl5 and SF6 to form, which deviate from the traditional octet rule. Expanding the valence shell is not possible for atoms in the second period since they lack an energetically accessible d-subshell. Therefore, these elements must obey the octet rule and cannot expand their valence shell beyond 8 electrons.
