Do you want to learn how to use the C1V1=C2V2 equation? This equation is used to calculate the unknown quantity of two solutions or mixtures that are proportional. It can be used for a number of purposes including mixing up different chemicals, creating dilutions, and calculating concentrations.
To understand this equation, let’s take a look at what each variable means. C1V1 represents the concentration and volume of the starting solution whle C2V2 represents the concentration and volume of the final solution. The total amount doesn’t change which means that the initial concentration multiplied by the initial volume must equal the final concentration multiplied by the final volume in order for this equation to work properly.
Now that we understand what each variable in this equation stands for, let’s take a look at how it can be used. One application of this equation is making dilute solutions from a stock solution. To do this, you would use the formula: C1V1 = C2V2 where V1 is equal to the volume of stock solution needed to make the new solution, C1 is equal to the concentration of stock solution, and V2 is equal to the final volume of new solution.
The dilution equation can also be used to figure out how much solvent needs to be added into a solution in order to dilute it. When adding more solvent into a solution, it will decrease its concentration but increase its overall volume. Therefore, you need to make sure that you have enough solvent available in order for this process to work properly.
These are just some examples of how you can use the C1V1=C2V2 equation in your daily life or in a laboratory setting. Understanding how concentrations and volumes are related will help you understand more complex equations as well as create accurate reactions with different chemicals and solutions!
The C1V1 C2V2 Equation
The C1V1 C2V2 equation is a formula used to calculate an unknown quantity when two solutions or mixtures are proportional. It states that the concentration or amount (C1) multiplied by the volume (V1) of the starting solution is equal to the concentration or amount (C2) multiplied by the volume (V2) of the final solution. In oher words, C1*V1=C2*V2. This equation can be used to find either a missing concentration or a missing volume. For example, if you know the starting concentration and volume and you need to find out what the final concentration and volume should be, you can use this equation to solve for either one.
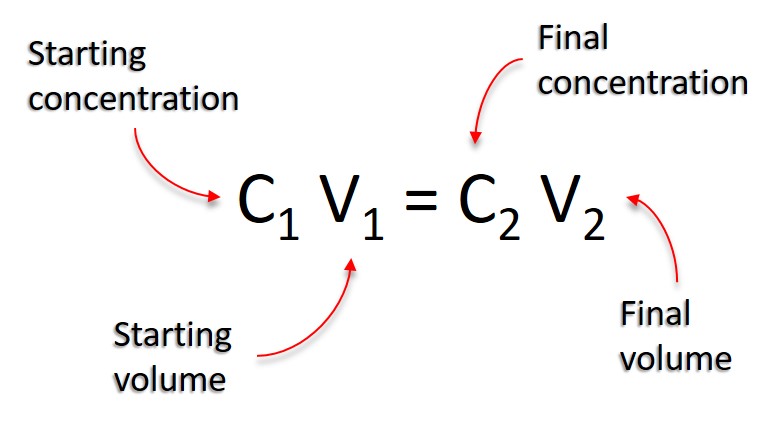
Understanding the Equation C1V1 = C2V2
The equation C1V1 equals C2V2 works because it is based on the law of conservation of mass. This law states that the total amount of mass in a closed system remains constant, regardless of any changes to the system. When two solutions are mixed together, their combined volume and concentration will remain the same. The equation C1V1 equals C2V2 simply expresses this fact by showing that the initial concentration multiplied by the initial volume is equal to the final concentration multiplied by the final volume.
Calculating V1 Dilution
To calculate the volume of stock solution (V1) needed to make a dilute solution, you need to use the formula C1V1 = C2V2. In this formula, C1 is the concentration of the stock solution, V2 is the final volume of the new solution and V1 is the volume of stock solution that needs to be added. To solve for V1, you will divide both sides of this equation by C2 and then multiply by V2. This gies you:
V1 = (C1/C2) * V2
For example, if you have a stock solution of 10% (C1) and you want to make 100 ml (V2) of a 4% dilution (C2), then your calculation would be:
V1 = (10/4)*100
Therefore, you would need to add 250 ml (V1) of stock solution in order to make 100 ml (V2) of 4% dilution.
The Dilution Formula
The dilution formula is used to calculate the amount of solvent needed to dilute a solution. It can be expressed as C1V1 = C2V2, whre C1 and V1 are the concentration and volume of the original solution, and C2 and V2 are the concentration and volume of the diluted solution. This formula can be used for diluting any type of solution, including solvents, acids, bases, and other mixtures. To use this formula, you need to know the initial concentration and volume of the original solution. Then you can calculate how much solvent needs to be added to obtain your desired final concentration.
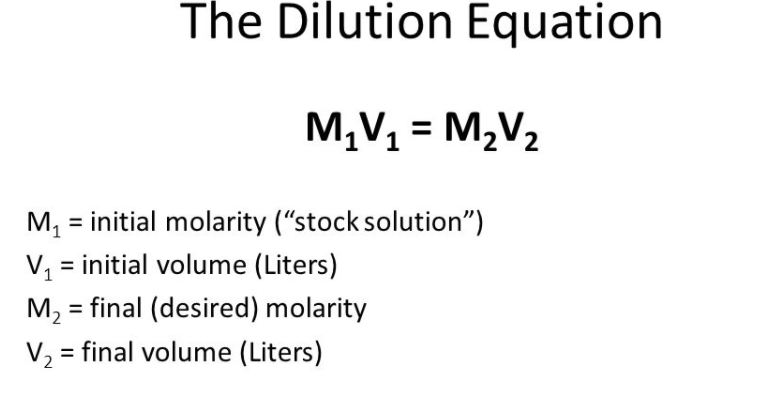
The Formula for M1v1 and M2v2
The m1v1 m2v2 formula is a type of dilution equation, and is also known as the M1V1M2V2 formula, or the dilution equation formula. This formula is used to calculate a missing value when two solutions of different molarities and volumes are mixed together. The equation works by multiplying the volume of one solution (V₁) by its molarity (M₁), and then dividing that result by the volume of the other solution (V₂). The resulting answer gives the molarity of the second solution (M₂).
Using C1V1 and C2V2 for Dilution
Yes, C1V1 = C2V2 can be used for dilution calculations. This formula is based on the principle of conservation of mass, where the amount of solute (C1) in a gien volume (V1) is equal to the amount of solute (C2) in a different volume (V2). To use this equation to calculate a dilution, you need to know the initial concentration and volume of the starting solution (C1 and V1), as well as the desired final concentration and volume (C2 and V2). By rearranging the equation, you can calculate what volume of the starting solution is needed in order to achieve the desired final concentration: V2 = (C1 x V1)/C2. Once you have your volumes calculated, you can then prepare your dilution by combining your starting solution with a suitable solvent.
The Purpose of Calculating Volume
The purpose of calculating volume is to measure the capacity of an object and determine how much space it occupies. Volume is used in a variety of contexts, from everyday tasks like measuring out ingredients for cooking or baking, to more scientific endeavors such as determining the density of a material or calculating the displacement of a liquid. Knowing the volume of an object can help us understand its properties and behavior, such as how much of a certin material it can hold or the amount of force required to move it. In addition, understanding the volume of an object can be important in engineering design and other projects that require precise measurements.
Calculating Concentration from a Dilution Ratio
To find the concentration from a dilution ratio, you need to express each dilution as a fraction and then multiply the concentration of the initial solution by the dilution factor used in each successive step. For example, if you are starting with a solution of 10% (concentration expressed as 0.1) and are making two successive dilutions of 1:2 and 1:4, the resulting concentration can be calculated using the following steps:
Step 1: Express each dilution ratio as a fraction. In this case, 1:2 is equivalent to ½ and 1:4 is equivalent to ¼.
Step 2: Multiply the initial concentration (0.1) by each successive dilution factor (½ and ¼). This givs us an answer of 0.05 for the final concentration of our solution.
Therefore, in this example, we can conclude that our resulting solution has a concentration of 5%.
What Does V1 Represent in M1V1 M2V2?
V1 is the initial volume of the solution before it was diluted. In the equation M1V1 = M2V2, V1 is multiplied by the initial concentration (M1) to equal the total amount of solute in the final solution after it has been diluted (M2V2). To calculate V1, you can rearrange the equation to solve for V1: V1 = M2V2 / M1.
Comparing V2 and V1 with T2 and T1
V2 V1 T2 T1 are all variables used in the formula for average acceleration, which is the rate of change of velocity over time. V2 and V1 represent the initial and final velocities, respectively, while T2 and T1 represent the initial and final times, respectively. The formula for average acceleration is given as:
Average Acceleration = (V2 – V1) / (T2 – T1)
This formula expresses how much the velocity has changed over a given period of time.
Comparing V1 and V2
V1 and V2 are two very important speeds in aviation. V1 is the speed at whih a pilot must make a decision to continue flight if an engine fails during takeoff. This speed is also known as the “commit to fly” speed as it marks the point of no return for continuing the flight. V2, on the other hand, is known as the takeoff safety speed and it is the speed at which an airplane will climb in the event of an engine failure after takeoff. It is essential that pilots become familiar with these two speeds, as they are key to a safe takeoff and flight.
Conclusion
In conclusion, the C1V1=C2V2 equation is a useful tool in helping to calculate an unknown quantity when two solutions or mixtures are proportional. This equation can be used to figure out the amount of solvent needed to dilute a solution, as well as determining the concentration of a given solution. C1 represents the concentration of the stock solution, V1 represents the volume of the stock solution needed to make up the new solution, C2 represents the newly created concentration, and V2 stands for the new volume of the solution. With all this information, one can easily solve for any unknowns with this equation.
