Hydrocarbons are organic compounds composed of only carbon and hydrogen. They are an important part of many everyday products, such as plastics and fuels. An important property of hydrocarbons is their polarity – or lack thereof.
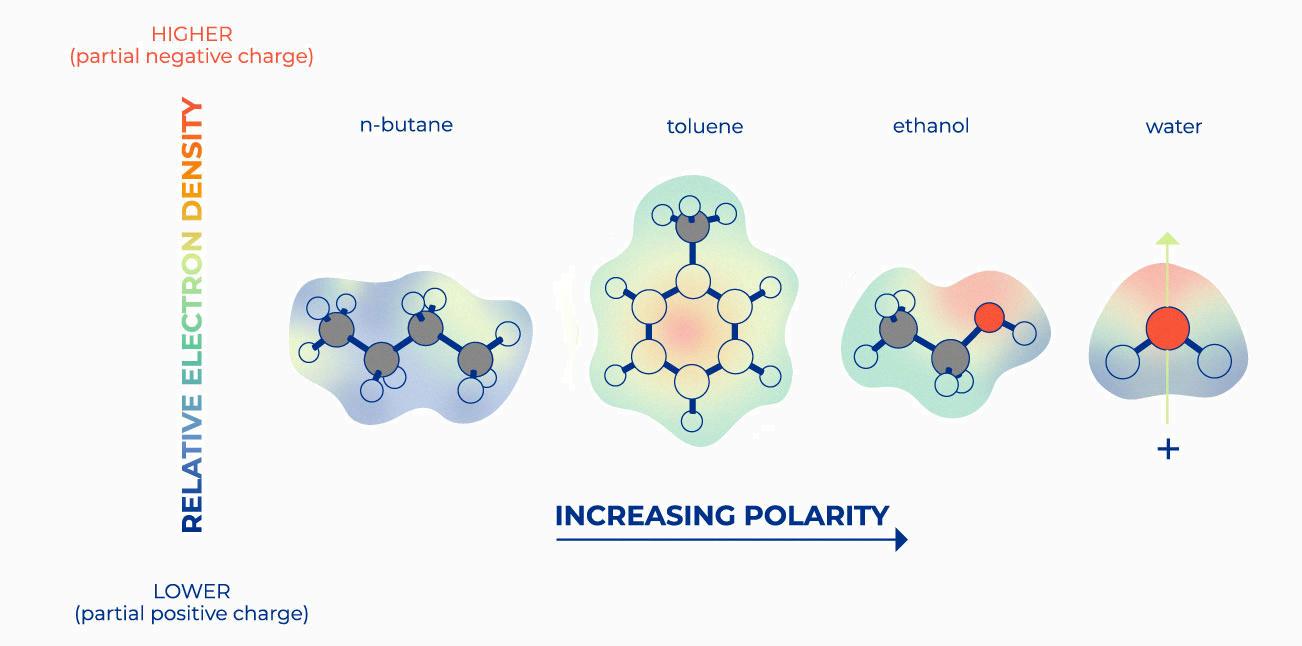
The polarity of a molecule is determined by the difference in electronegativity between the atoms that make up the molecule. The greater the difference in electronegativity, the more polar the molecule is. In hydrocarbons, the difference in electronegativity between carbon and hydrogen is very small (EN = 0.40), which means that hydrocarbons are nonpolar molecules. This means that they do not dissolve in polar solvents such as water, but instead repel them.
The reason why hydrocarbons are nonpolar molecules is because of their molecular structure. Each carbon atom has four electrons in its outer shell, which it shares equally with hydrogen atoms to form single covalent bonds – also known as sigma bonds. These covalent bonds have no net dipole moment, meaning that there is no separation of charge along the chain, resulting in a non-polar molecule.
In contrast to hydrocarbons, carboxylic acids contain two oxygen atoms which each have two electron clouds that give these regions around the molecule a negative charge – known as a dipole moment – making them polar molecules. Polar molecules can dissolve in other polar solvents such as water due to their different charges being attracted to one another – whereas nonpolar molecules repel each other due to having equal charges on both sides of the bond.
It is therefore important to understand the differences between polar and nonpolar molecules when choosing materials for certain applications such as chemical synthesis or engineering processes; understanding these differences can help you make informed decisions abut what material you need for your project or experiment.
Are Hydrocarbons Nonpolar?
No, not all hydrocarbons are nonpolar. While many hydrocarbons have a very small dipole moment due to the small difference in electronegativity between carbon and hydrogen atoms, there are some exceptions. For example, if a hydrocarbon has more than one double or triple bond, the molecule may be polar due to the more significant difference in electronegativity between the atoms involved in the bond. Additionally, if a hydrocarbon contains an atom other than carbon and hydrogen, such as nitrogen or oxygen, it may also be polar. In thee cases, the dipole moment of the molecule is determined by the relative electronegativities of all of its atoms.
The Nonpolar and Hydrophobic Nature of Hydrocarbons
Hydrocarbons are nonpolar and hydrophobic because the difference between the electronegativities of carbon and hydrogen is small (EN = 0.40). This means that the electrons in a hydrocarbon molecule are shared equally between the atoms, which results in a nonpolar molecule with no partial charges. Since tese molecules lack any significant electrical charge, they do not interact strongly with water molecules or other polar compounds. As a result, hydrocarbons don’t dissolve in polar solvents such as water, making them hydrophobic.
The Nonpolar Nature of Hydrocarbon Tails
Hydrocarbon tails are composed of only carbon and hydrogen atoms, which have very similar electronegativities. This results in the electrons being shared equally between the atoms, creating a molecule with no net charge – making it nonpolar. The lack of polarity means that hydrocarbon tails cannot form hydrogen bonds with water molecules and other polar molecules, so they remain neutral and insoluble in water.
Are Alkenes Polar or Nonpolar?
No, all alkenes are not nonpolar. Due to the unequal sharing of electrons in their covalent bonds, some alkenes can have a slightly polar nature. This is because the electrons that form the covalent bond between two atoms of different elements can be unequally shared between them due to differences in electronegativity. This creates an area of partial positive charge around one atom and partial negative charge around another, giving the molecule a slight dipole moment and making it slightly polar.
Identifying Polar and Nonpolar Compounds
When determining whether a compound is polar or nonpolar, it is important to consider the electronegativity of the atoms that make up the compound. The greater the difference in electronegativity between two atoms in a bond, the more polar that bond will be. If the difference in electronegativity is greater than 0.4, then the bond is considered to be polar. On the other hand, if the difference in electronegativity is less than 0.4, then the bond is essentially nonpolar. Thus, if there are no polar bonds present in a molecule, then we can say that this molecule is nonpolar.
Source: labsociety.com
Hydrophobicity and Hydrophilicity of Hydrocarbons
No, not all hydrocarbons are hydrophobic. While many hydrocarbons are non-polar and thus hydrophobic, some hydrocarbons contain functional groups that make them polar and therefore hydrophilic. Examples of such hydrocarbons include alcohols, carboxylic acids, and amines. As a general rule, the more complex the hydrocarbon is (i.e. the more functional groups it contains), the more likly it is to be hydrophilic.
Are Hydrocarbons Hydrophobic?
No, not all hydrocarbons are hydrophobic. Some hydrocarbons contain polar functional groups, such as alcohols or carboxylic acids, whih can interact with water to form hydrogen bonds. These molecules have a higher solubility in water than nonpolar hydrocarbons and are therefore considered to be hydrophilic. Additionally, some hydrocarbon molecules may contain heteroatoms, such as nitrogen or oxygen, which can form hydrogen bonds with water molecules and make the molecule more soluble in water.
Hydrophobicity of Hydrocarbons
Yes, hydrocarbons are generally hydrophobic. This means that they repel water and do not mix with it. Hydrocarbons are composed solely of hydrogen and carbon atoms, so their molecules are non-polar which prevents them from forming hydrogen bonds with water molecules. This makes them hydrophobic and cases them to form a separate layer when mixed with water.
Non-Polar Hydrocarbons
Hydrocarbons are organic molecules that contain only carbon and hydrogen atoms. Nonpolar hydrocarbons are molecules that have the same number of electrons on each side of the molecule, which makes the molecule not charged. Examples of nonpolar hydrocarbons include methane (CH4), ethane (C2H6), propane (C3H8) and butane (C4H10). These molecules do not have any net dipole moment and therefore can be classified as non-polar molecules.

Source: polarconnection.org
Is a Hydrocarbon Tail Polar or Nonpolar?
The hydrocarbon tail of a hairpin is non-polar. This is because the tail is made up of two long chains of carbon and hydrogen atoms bound together by chemical bonds. These atoms have a strong affinity for each other, which creates an overall non-polar environment in which electrons are evenly distributed among the atoms, making it unable to form any hydrogen bonds or other polar interactions.
Why Alkanes Are Non-Polar
Alkanes are non-polar compounds because they contain only carbon-carbon and carbon-hydrogen bonds. The electronegativity values of both carbon and hydrogen are very similar, so teir C—H bonds are essentially nonpolar. This means that the molecules do not possess any permanent dipoles, and can only interact through weak London forces. As a result, alkanes have low solubility in polar solvents, such as water, and are generally insoluble in them.
Conclusion
In conclusion, hydrocarbons are nonpolar molecules due to the small difference in electronegativity between carbon and hydrogen. This means that hydrocarbons do not dissolve in polar solvents such as water and have a consistent electron density aong the chain. However, carboxylic acid groups within a hydrocarbon molecule can create regions with a negative charge, which can affect its overall polarity.
