Carbohydrates are a class of molecules that are present in the majority of foods and are important to the body’s energy production. But what you may not know is that carbohydrates can be either hydrophilic (water-soluble) or hydrophobic (water-insoluble).
The structure of carbohydrates determines whether they are hydrophilic or hydrophobic. Most carbohydrates contain several -OH functional groups whih make them polar molecules, meaning they dissolve well in water and have a positive charge. This makes them hydrophilic, meaning they can form hydrogen bonds with water and other polar molecules, allowing them to dissolve in water. An example of a hydrophilic carbohydrate is glucose, which dissolves easily in water.
On the other hand, some carbohydrates such as lipids are nonpolar and thus, not soluble in water. Lipids are also referred to as “hydrophobic” because they repel water instead of dissolving like polar molecules do. Lipids contain fatty acids instead of -OH functional groups, making them nonpolar and unable to form hydrogen bonds with water. An example of a hydrophobic carbohydrate is triglycerides, which do not mix with water but rather clump together in tiny droplets that float on the surface.
In conclusion, carbohydrates can be either hydrophilic or hydrophobic depending on their structure. Hydrophilic carbohydrates such as glucose dissolve easily in water while hydrophobic carbohydrates such as lipids do not mix with water but float on its surface instead. Knowing the difference between these two types of carbohydrates will help you make better food choices for your health and overall wellbeing!
Hydrophobicity of Carbohydrates
Carbohydrates are generally hydrophilic due to their polar nature. They contain several –OH functional groups, which can interact with water molecules and form hydrogen bonds. This makes them soluble in water and therefore hydrophilic. Hydrophobic molecules, on the oter hand, do not dissolve in water because they lack these polar functional groups and cannot form hydrogen bonds with water molecules.
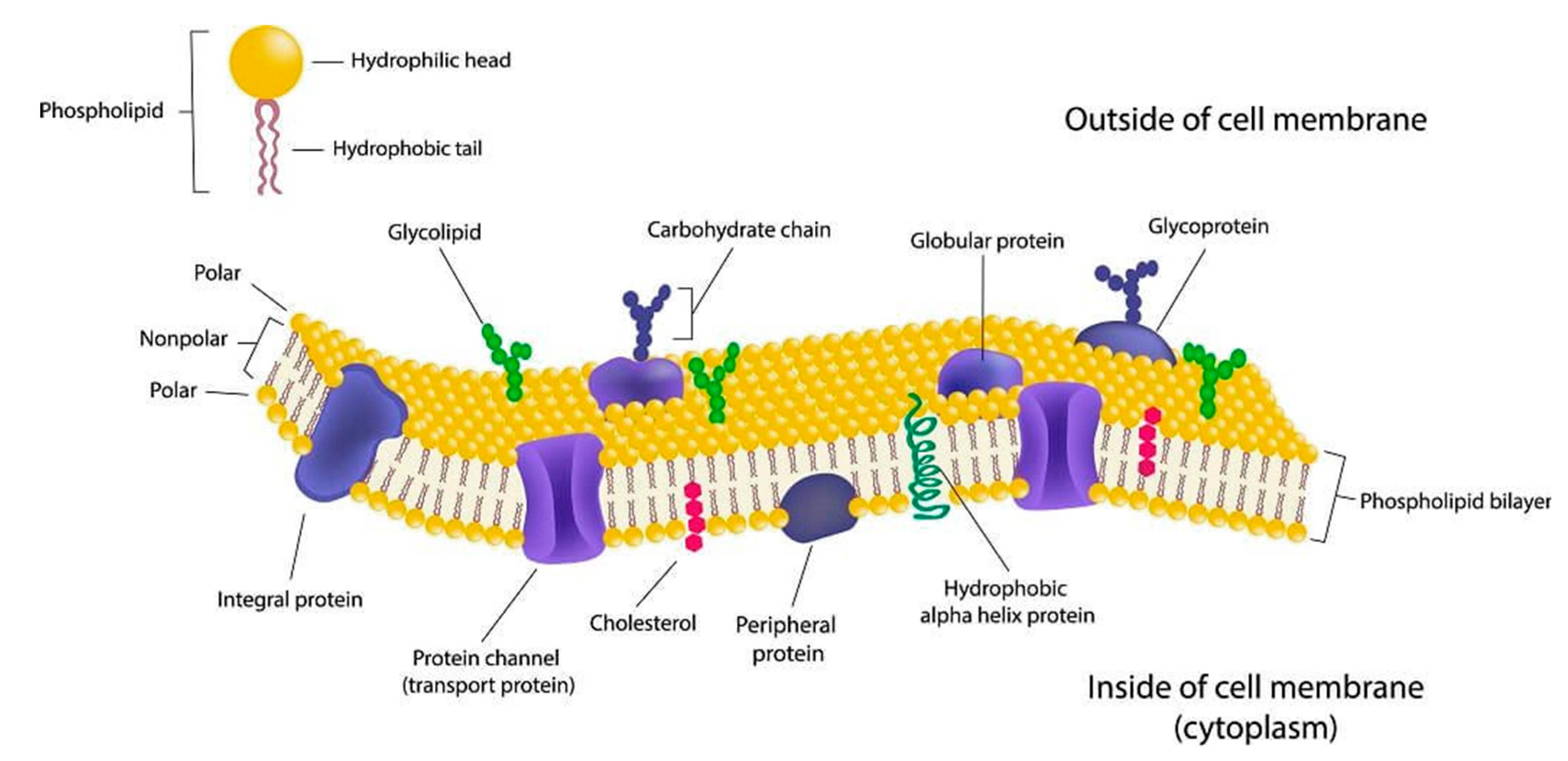
Source: mdpi.com
Are Carbohydrates Hydrophobic or Hydrophilic?
Carbohydrates are hydrophilic, meaning they are highly soluble in water. They have polar molecules with many hydroxyl groups that attract water molecules and easily dissolve in it. Lipids, on the other hand, are hydrophobic, meaning they do not dissolve in water because they are nonpolar molecules without any polar functional groups.
Hydrophobicity of Carbohydrates and Lipids
No, carbohydrates and lipids are not hydrophobic. Lipids are hydrophobic molecules, meaning they do not interact with water and tend to be insoluble in it. Carbohydrates, on the other hand, are hydrophilic molecules that are able to form hydrogen bonds with water molecules and tend to be soluble in it.
Can Carbohydrates Dissolve in Water?
Yes, carbohydrates will dissolve in water. Carbohydrates are hydrophilic molecules, meaning they are attracted to water molecules and dissolve when placed in water. The solubility of carbohydrates depends on the type of sugar molecule; for example, monosaccharides such as glucose and fructose dissolve easily in water, wheeas disaccharides such as sucrose and lactose require more energy to dissolve. In addition, some polysaccharides like cellulose and starch are insoluble in water due to the large size of their molecules.
Is Protein Hydrophobic Or Hydrophilic?
Proteins are generally considered to be amphipathic molecules, meaning they have both hydrophilic and hydrophobic components. The amino acid side chains that make up proteins can be eithr polar (hydrophilic) or non-polar (hydrophobic). Polar side chains are attracted to water and tend to be found on the outside of the protein. Non-polar side chains, on the other hand, are repelled by water and will typically be found inside the protein structure. Generally speaking, proteins have a net charge that is either slightly positive or slightly negative depending on the types of amino acids present. This charge will determine how proteins interact with the surrounding environment, making them either hydrophilic or hydrophobic in nature.

Hydrophobicity of Glucose
Glucose is a highly hydrophilic molecule, meaning that it has a strong affinity for water. It is composed of six carbons, twelve hydrogens, and six oxygens, with the oxygen atoms forming hydrogen bonds with the surrounding water molecules. Glucose is also a polar molecule due to its molecular structure, whch allows it to interact with other polar molecules like water. As such, glucose readily dissolves in water and is considered hydrophilic.
Identifying Hydrophobic and Hydrophilic Properties
The way to determine if a material is hydrophobic or hydrophilic is to observe its interaction with water. Hydrophilic materials will have water spread across them, maximizing contact, while hydrophobic materials will cause the water to form into droplets and roll off the surface. Additionally, a material can be tested by adding a drop of water to it and observing what happens. If the drop spreads out across the surface, it is hydrophilic; wheres if the drop beads up and rolls off, it is hydrophobic.
What Type of Molecule is Hydrophobic?
Hydrophobic is not a carbohydrate, lipid, or protein. It is an adjective that describes the physical property of molecules that are not attracted to water. Lipids are generally hydrophobic because the non-polar covalent bonds linking their carbons and hydrogens aren’t attracted to the polar bonds of water. The four major groups of lipids include fats, phospholipids, waxes and steroids.
Are Carbohydrates Polar or Nonpolar?
No, not all carbohydrates are nonpolar. Carbohydrates are a broad class of molecules containing carbon, hydrogen, and oxygen. These molecules can range from simple three-carbon sugars to complex chains of hundreds of atoms. While many carbohydrates are nonpolar, some have highly polar components such as alcohols and amines that give them a net polar character. In fact, the sugar molecules that make up the majority of dietary carbohydrates are highly polar due to the presence of hydroxyl groups (-OH). As such, these molecules dissolve readily in water and other polar solvents.

What Lipids Are Hydrophobic?
Lipids are hydrophobic molecules that are not soluble in water. Examples of hydrophobic lipids include fatty acids, triglycerides, waxes, and sterols such as cholesterol. Fatty acids are composed of long, nonpolar hydrocarbon chains that do not interact with water molecules. Triglycerides, which are molecules composed of fatty acids and a glycerol backbone, also contain nonpolar hydrocarbon tails and are therfore not soluble in water. Waxes are esters of long-chain fatty acids and long-chain alcohols and contain both polar and nonpolar components; however, the nonpolar components dominate in waxes making them largely hydrophobic. Sterols such as cholesterol contain multiple rings of hydrocarbons and alcohol groups that also make them hydrophobic. Finally, phospholipids contain two hydrocarbon tails and a polar head group; however, the hydrocarbon tails dominate the structure of phospholipids making them largely hydrophobic as well.
Hydrophobicity of Lipids
Lipids are generally hydrophobic, meaning that they do not dissolve in water. This is because lipids are composed mainy of hydrocarbons, which are nonpolar molecules that do not interact well with water molecules. Lipids have a range of structural features and functions in organisms, but their hydrophobic nature is the defining feature that separates them from other groups of molecules like carbohydrates and proteins, which are both hydrophilic. As such, lipids will not typically dissolve in aqueous solutions and instead tend to form relatively insoluble aggregates or emulsions.
The Hydrophilic Nature of Glucose
Glucose is hydrophilic (water-loving) due to its molecular structure and the way it interacts with water molecules. The glucose molecule contains several oxygen atoms that can form strong hydrogen bonds with the oxygen and hydrogen atoms in water molecules. This allows glucose to form multiple hydrogen bonds with a single water molecule, making it more likely to be soluble in water. Additionally, the hydroxyl (OH) groups of glucose make it more likely to interact with water due to the polar nature of the molecules involved. These factors combined create an environment in wich glucose molecules are attracted to each other and therefore dissolve easily in water.
Why Carbohydrates Are Insoluble in Water
Carbohydrates are insoluble in water becuse of their chemical structure. Carbohydrates consist of long chains of molecules, known as polysaccharides, which cannot be broken down by the molecules of water. The chemical bonds between the molecules of the carbohydrate are so strong that they do not easily dissolve in the polar environment of water. As a result, carbohydrates remain insoluble in water and form a suspension or colloid.
Carbohydrates That Do Not Dissolve in Water
Cellulose is the carbohydrate that does not dissolve in water. It is a polysaccharide, composed of many glucose monomers, wich are connected by strong chemical bonds that make it insoluble in water. Unlike other polysaccharides such as starch, cellulose cannot be broken down into its component monomers and therefore stays solid when in contact with water. Cellulose is found in plant cell walls and the structural support of plants and trees, making it an important component of the food chain.
Are Carbohydrates Polar or Nonpolar?
Carbohydrates are generally classified as polar molecules. This is because the oxygen and hydrogen atoms in the carbohydrate structure form hydrogen bonds, which create a net dipole moment across the molecule. This means that one end of the molecule is slightly more positive than the other end, making it polar. Polar molecules interact with water molecules throgh hydrogen bonding, allowing them to dissolve in water, while nonpolar molecules such as hydrocarbons do not mix with water and do not dissolve. Therefore, carbohydrates are considered polar molecules.
Conclusion
In conclusion, carbohydrates are hydrophilic and not hydrophobic. This is because they contain several –OH functional groups, which makes them polar molecules and therefore able to form hydrogen bonds with water. Carbohydrates also dissolve easily in both water and alcohol, making them soluble in aqueous environments. Therefore, carbohydrates cannot be classified as hydrophobic as they do not repel water but instead interact with it.
