Antimony tribromide is a chemical compound that has a chemical formula of SbBr3. It is composed of antimony and bromide ions in an equal ratio. This compound is often used in chemistry experiments due to its relatively low toxicity, but it’s important to be aware of its potential hazards when handling it.
Antimony tribromide can be found naturally occurring in the environment, though its most common use is as a laboratory reagent. In organic chemistry, it is often used as a catalyst for various reactions. It can also be used as an oxidizing agent in some circumstances. Antimony tribromide is also known to be usful for making organobromine compounds, which can then be used for different purposes such as pharmaceuticals and biochemistry research.
When handling this chemical compound, safety precautions should always be taken. As with any other substance, protective clothing and eyewear should always be worn when working with antimony tribromide. The compound can release toxic bromine gas if heated or mixed with certain substances and should not be heated above 200 degrees Celsius or mixed with acids or bases without proper protective gear and ventilation. In addition, contamination of skin or eyes should be avoided at all costs sice antimony tribromide is corrosive and may cause irritation or burns if contact occurs.
All in all, antimony tribromide is an important reagent in organic chemistry due to its various uses and relatively low toxicity level compared to other compounds of similar nature. However, safety precautions should always be taken when handling this substance snce it can still pose a health risk if not dealt with properly.
The Name of Antimony Tribromide
The chemical name for Antimony tribromide is Tribromostibine, or more specifically the molecular formula for it is Br3Sb or SbBr3. It is also known by the CAS number 7789-61-9 and other synonyms such as Antimony(III) Bromide and Antimony tribromide. It can be found in PubChem with the CID 24615.
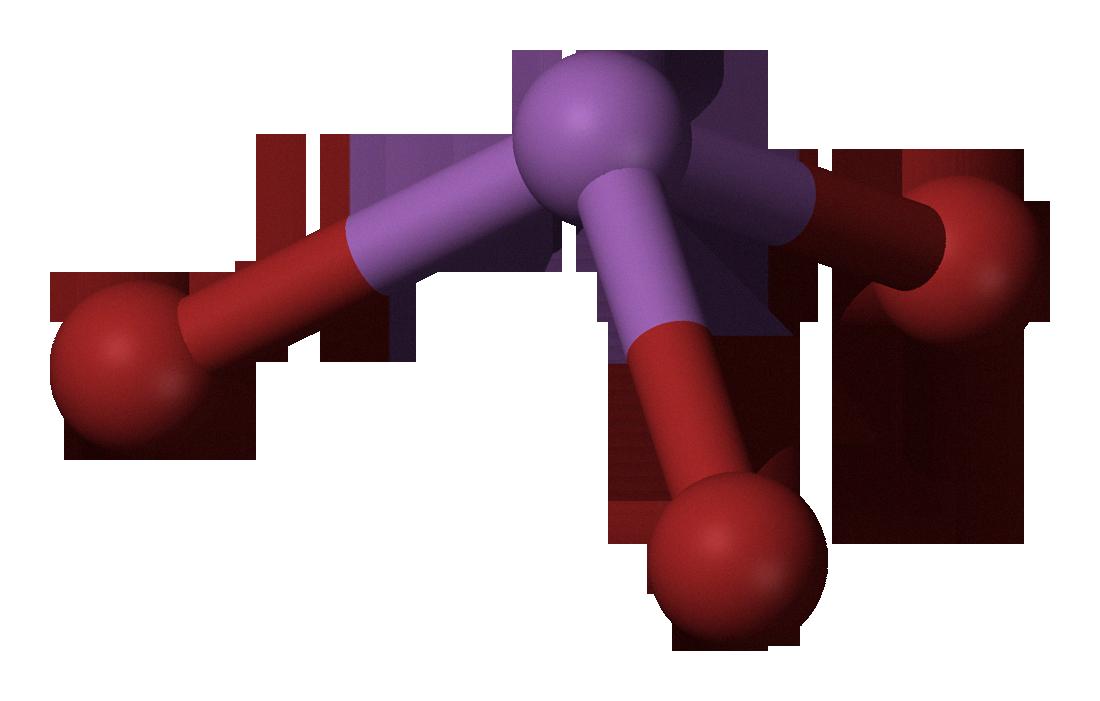
Source: commons.wikimedia.org
Is Antimony Tribromide an Ionic Compound?
Yes, antimony tribromide is an ionic compound. It is composed of ions of antimony (Sb3+) and bromide (Br-). Ionic compounds are formed when a metal transfers electrons to a nonmetal, forming positive and negative ions that attract each other. As such, the antimony and bromine atoms in antimony tribromide are held together by the electrostatic forces of their opposite charges.
Formula for Antimony III Chloride
The formula for antimony III chloride is Sb2Cl6. It is an inorganic compound composed of two antimony atoms and six chlorine atoms, forming a hexagonal structure. Antimony III chloride has a molar mass of 306.44 g/mol and a melting point of 297°C. The compound is soluble in water, producing hydrochloric acid and antimony trichloride when dissolved.
Formula for Antimony III Perphosphate
The formula for antimony III perphosphate is O4PSb, also kown as antimony(3+) phosphate. This compound has a molecular weight of 216.73 and is composed of two components – antimony (CID 5354495) and phosphoric acid (CID 1004). It is also referred to by its systematic name, 2,4,5-trioxa-1lambda5-phospha-3-stibabicyclo[1.1.1]pentane 1-oxide, or by its CAS number 12036-46-3.
What is NBr3?
Nitrogen tribromide (NBr3) is an explosive, deep-red and volatile chemical compound. It was not isolated until 1975, and is sometimes referred to as dibromoazane or nitrogen bromide. It has the formula NBr3, and its boiling point is 10°C. When exposed to heat, nitrogen tribromide decomposes into nitrogen, bromine and hydrogen bromide. It is an extremely dangerous compound and should be handled with extreme caution.
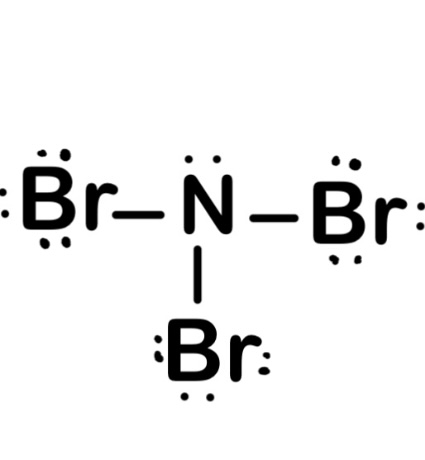
Is NBr3 an Ionic or Covalent Bond?
Nitrogentribromide (NBr3) is a covalent bond because the electronegativity difference betwen nitrogen and bromine is not great enough to form an ionic bond. The electronegativity of nitrogen is 3.04 and that of bromine is 2.96, which results in a difference of only 0.08, indicating that the bond between these two non-metal atoms will be covalent. Covalent bonds occur when atoms share electrons in order to fill their outer shell and achieve stability, while ionic bonds occur when one atom transfers electrons to another atom resulting in opposite charges that are attracted to each other.
Is Brbr an Ionic or Covalent Compound?
The Br-Br bond is a covalent bond, which means it involves the sharing of electrons between two atoms. Specifically, it is a nonpolar covalent bond, which means that there is no electronegativity difference between the two bonded atoms. This means that the electrons in the bond are shared equally between the two atoms. As a result, there are no charged ions present, so this is not an ionic bond.
Bonding Type of NBr3
Answer: NBr3 consists of covalent bonding. Covalent bonds occur when two nonmetal atoms share electrons to form a chemical bond. In the case of NBr3, the nitrogen atom shares its electrons with the thee bromine atoms, forming a molecule with three covalent bonds.
Chemical Name of Na3Bo3
The chemical name of Na3BO3 is trisodium borate. It is an inorganic compound composed of three sodium cations (Na+) and one borate anion (BO3-). The compound has a molar mass of 137.83 g/mol and a density of 2.44 g/cm3. It can be found in the form of white powder or crystals, whch are insoluble in water but soluble in alcohols and other polar solvents. Trisodium borate has many uses, including as a pH buffer, cleaning agent, and flame retardant.
The Name of PO3 3
The name of the po3 3 ion is phosphite (3-). Phosphite is a trivalent inorganic anion obtained by removal of all tree protons from phosphorous acid. It has a formal charge of -3, and a heavy atom count of 4, as determined by PubChem. This ion plays an important role in many biochemical processes, such as DNA and RNA synthesis, metabolism, and energy production.
The Origin of the Name ‘Antimony’
Antimony derives its name from the Greek words ‘anti’ and ‘monos’, which mean “not alone” or “not one”. This is because it was found in many compounds. The symbol Sb for antimony comes from the Greek word ‘stibium’, which means “mark”. This is because antimony was traditionally used as a blackening agent to darken eyebrows and eyelashes.

The Name of H3P03
The name of H3P is Phosphine-d. It is an inorganic compound with the chemical formula H3P and molecular weight of 33.998 g/mol. It has the PubChem CID 139504 and DTXSID20159584, and is sometimes referred to as phosphane-d or dimethylphosphane. The compound was fist created in 2005, and was last modified in 2022.
What Is the Name of Hg3 PO4 2?
Hg3(PO4)2 is the chemical compound known as mercury(II)phosphate. It is an inorganic salt composed of three mercury cations and two phosphate anions, which form a crystal structure with a density of 5.45 g/cm3. Mercury(II)phosphate is insoluble in water, but soluble in acids. It has a variety of applications in industries such as electroplating, pharmaceuticals, and oher chemical processes.
Conclusion
In conclusion, antimony tribromide is a chemical compound with the molecular formula Br3Sb or SbBr3. It is composed of antimony and bromide ions, and its related compound antimony trichloride has the formula SbCl3. Antimony(3+) phosphate has the formula O4PSb and is composed of antimony and phosphoric acid. All of these compounds are important in varius industrial processes, making them essential components in modern chemistry.
