Chlorine (Cl) is a chemical element that belongs to Group 17 of the periodic table, also known as the halogens. The halogens are a group of highly reactive non-metallic elements that share similar chemical properties. They are located on the left side of the noble gases in the periodic table, and include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Chlorine is a greenish-yellow gas that is highly toxic and corrosive. It is not found in nature as an element, but rather as a compound, most commonly in the form of chloride salts such as sodium chloride (table salt) and potassium chloride. Chlorine is used in a variety of industrial processes, including the production of plastics, solvents, and bleach.
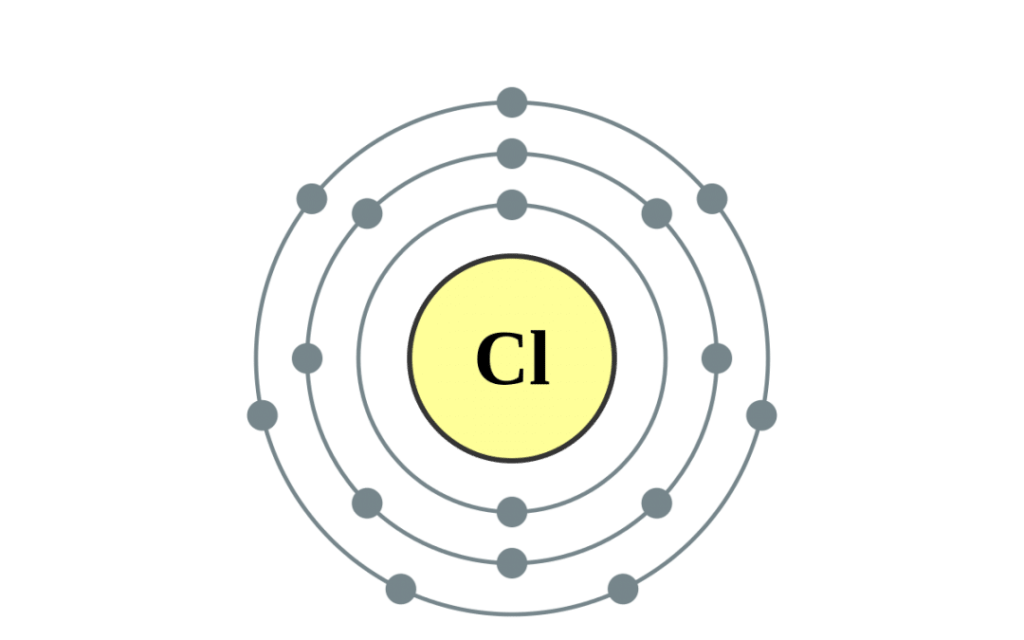
Like the other halogens, chlorine has sven valence electrons in its outermost shell, which makes it highly reactive. It readily forms compounds with other elements, particularly metals. Chlorine is a powerful oxidizing agent, meaning that it can easily donate electrons to other atoms or molecules. This property makes it useful in disinfecting water and killing bacteria and other pathogens.
Chlorine has many applications in everyday life. It is widely used as a disinfectant in swimming pools and drinking water, as well as in the production of paper, textiles, and pharmaceuticals. It is also used to purify metals, as a bleaching agent for textiles and paper, and as a component in many household cleaning products.
Chlorine is indeed a halogen, one of the most reactive and toxic elements in the periodic table. As a member of Group 17, it shares many chemical properties with other halogens, including its ability to readily form compounds with other elements. Despite its hazards, chlorine has many important uses in industry and everyday life.
Is Class A a Halogen or Noble Gas?
Chlorine (Cl) is an element that belongs to the halogen group. The halogen group consists of five oher elements, namely fluorine (F), bromine (Br), iodine (I), astatine (At), and tennessine (Ts).
Halogens are highly reactive nonmetals that have seven electrons in their outermost shell. They readily form compounds with other elements, especially metals, to achieve a stable electron configuration. Halogens are known for their strong oxidizing ability, which makes them useful in many industrial and biological processes.
On the other hand, noble gases are a group of elements that include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). These elements have a complete outermost shell, which makes them highly stable and non-reactive.
Chlorine (Cl) is a halogen, not a noble gas.
Is Class A a Transition Metal or a Halogen?
Chlorine (Cl) is a halogen and not a transition metal. Halogens are a group of highly reactive nonmetallic elements that are found in group 17 of the periodic table. They have sven valence electrons in their outermost shell, making them highly reactive and able to form compounds with most other elements.
In contrast, transition metals are found in the middle of the periodic table, in groups 3-12. They are known for their ability to form complex ions and compounds, as well as their metallic properties such as high conductivity and luster.
To summarize, chlorine is not a transition metal, but rather a halogen.
Periodic Group of Chlorine
Chlorine, denoted by the chemical symbol Cl, is a highly reactive non-metal element that belongs to Group 17 of the periodic table. This group is also known as the halogens and includes elements such as fluorine, bromine, iodine, and astatine.
The halogens are highly reactive due to their seven valence electrons, which makes them one electron short of having a full outer shell. As a result, they tend to form compounds with other elements to gain the missing electron and achieve a stable electron configuration.
Chlorine is not found in its elemental form in nature, but rathr as a compound, due to its reactivity. The most common compound of chlorine is salt, or sodium chloride (NaCl), which is abundant in seawater and is widely used in food preservation and seasoning. Other important chlorine compounds include potassium chloride (KCl), sylvite, and carnallite, which are used in fertilizers and water treatment.
Chlorine is a halogen element that belongs to Group 17 of the periodic table. It is highly reactive and forms compounds with other elements, and is not found in its elemental form in nature. Its most common compound is salt, and it is also used in various other compounds such as potassium chloride, sylvite, and carnallite.
Group Number of Halogens
Halogens are often referred to as Group 17 elements on the periodic table. This is because they are located in the seventh column or group from the left-hand side of the table. The elements in this group have similar properties such as being non-metallic, highly reactive, and able to form compounds with other elements (especially metals) easily.
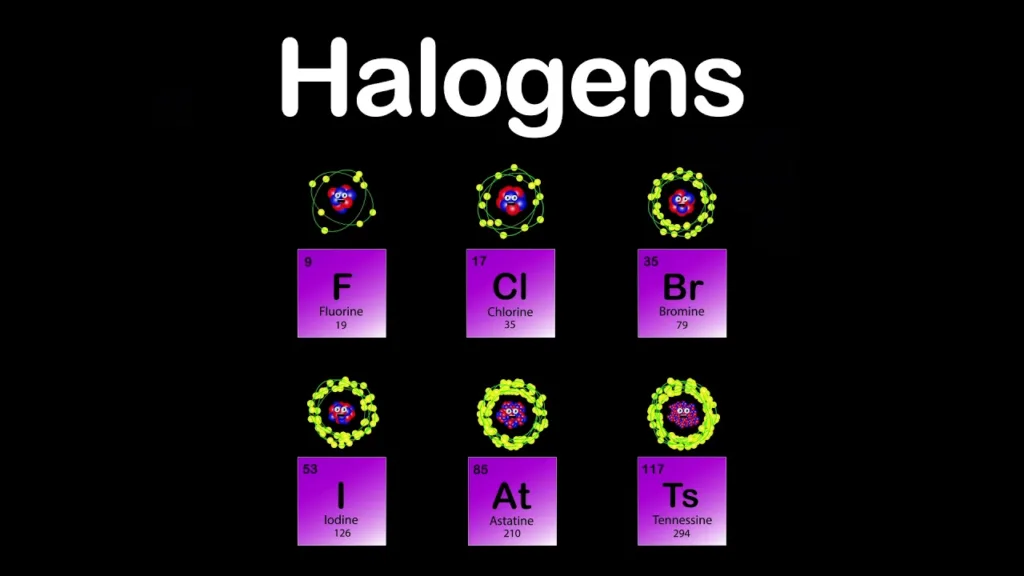
The halogen group is made up of five elements, namely fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These elements are all toxic and have unique physical and chemical properties. For instance, fluorine is a pale yellow gas that is highly reactive and can form compounds with almost all other elements. Chlorine, on the other hand, is a greenish-yellow gas that is commonly used as a disinfectant and bleach. Bromine is a reddish-brown liquid that can easily evaporate to form a gas, wile iodine is a dark gray solid that can sublimate to form a purple gas.
The halogens have a unique electronic configuration, with seven valence electrons in their outermost shell. This makes them highly reactive and able to easily gain or lose one electron to form ionic compounds. Halogens can also form covalent compounds by sharing electrons with other non-metallic elements.
To sum up, halogens are Group 17 elements located on the periodic table. They are highly reactive and toxic non-metals that can easily form compounds with other elements. Fluorine, chlorine, bromine, iodine, and astatine are the five elements in this group, each with unique physical and chemical properties.
Conclusion
Chlorine (Cl) is indeed a halogen, belonging to Group 17 of the periodic table. It is a highly reactive nonmetallic element, and is not found in nature as an element, but only as a compound. Chlorine is widely used in various industrial applications, including water treatment, paper production, and chemical manufacturing. Its reactivity makes it a valuable component in many chemical reactions, but also poses potential health and environmental risks if not handled properly. Understanding the properties and characteristics of halogens, including chlorine, is crucial for scientists, researchers, and industrial professionals working in a variety of fields.
