In the field of chromatography, the concept of Height Equivalent to a Theoretical Plate (HETP) is a crucial factor in determining the efficiency of a separation process. HETP is a measure of the efficiency of a column in separating two or more components in a mixture. It is a key parameter that helps researchers optimize the separation process and improve the quality of their results.
The HETP is defined by the equation HETP = σ2/L, where σ is the standard deviation and L is the distance traveled. This value represents the height of a theoretical plate that would provide the same separation efficiency as the actual column. The HETP value is inversely proportional to the number of theoretical plates in a column. Therefore, a low HETP value indicates a high number of theoretical plates and a more efficient separation process.
The HETP value is an important criterion for selecting and optimizing the separation process for different types of compounds. A low HETP value is desirable because it indicates a more efficient separation process with fewer theoretical plates. This means that the separation process is faster and more accurate, resulting in a better quality of separation.
The HETP value can be measured over time to monitor the performance of the column. This is important because the HETP value can change over time due to factors such as column degradation, canges in the mobile phase or stationary phase, or variations in the flow rate. By monitoring the HETP value, researchers can identify potential issues with the column and make adjustments to improve its efficiency.
To normalize the HETP value across columns of different sizes, the length of the column is divided by the number of theoretical plates (N) to provide the HETP value. This allows researchers to compare the efficiency of columns of different sizes and optimize their separation process accordingly.
HETP is a crucial parameter in chromatography that helps researchers optimize the separation process and improve the quality of their results. A low HETP value indicates a more efficient separation process with fewer theoretical plates, resulting in a faster and more accurate separation process. By monitoring the HETP value over time, researchers can identify potential issues with the column and make adjustments to improve its efficiency.
What is the HETP Formula?
The HETP formula is a mathematical equation used in chromatography to measure the efficiency of a column in separating compounds. HETP stands for “height equivalent to a theoretical plate” and is a measure of the average distance a compound travels through the column befre being separated from other compounds.
The formula for HETP is HETP = σ 2/L, where σ is the standard deviation of the compound’s retention time and L is the length of the column. The standard deviation measures the degree of variation in retention times for a particular compound. A smaller standard deviation indicates a more efficient separation process, as the compound is consistently retained and released at the same point in the column.
The HETP formula is useful for determining the optimal column length for a particular separation process. A shorter column length generally results in a lower HETP value, indicating a more efficient separation. However, other factors such as flow rate, column packing material, and compound properties can also affect separation efficiency.
The HETP formula is a key tool in chromatography for assessing the efficiency of a separation process and determining the optimal column length for a particular application.
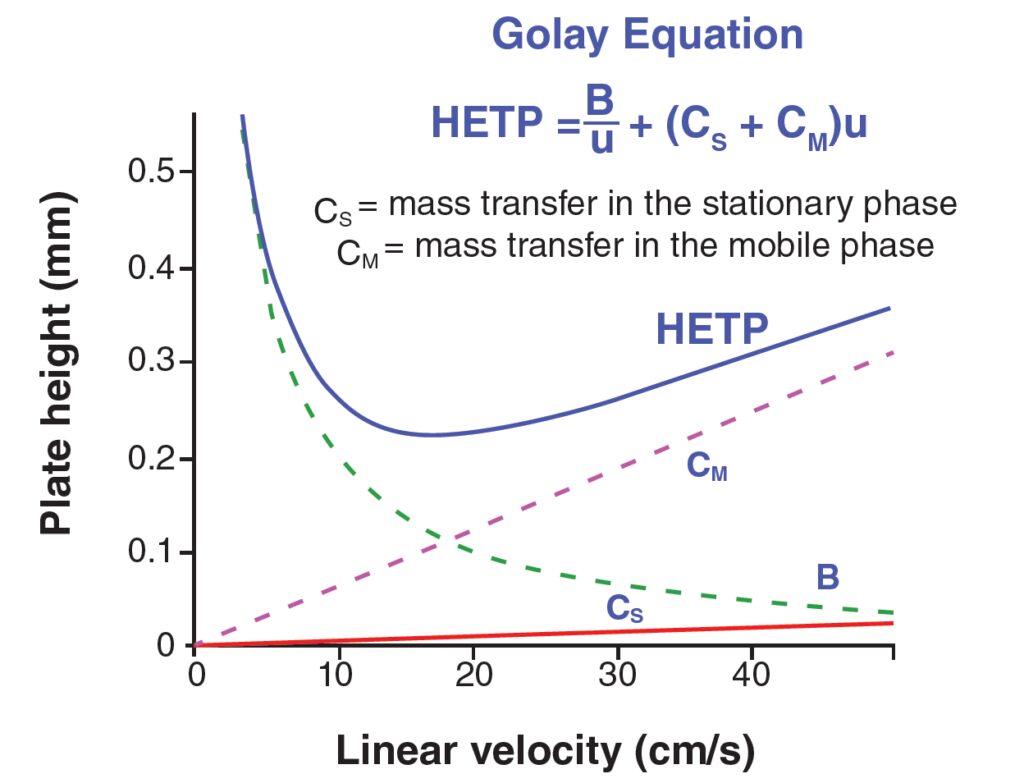
Understanding the Meaning of Hetp Value
In chromatography, the height equivalent to theoretical plate (HETP) is a measure of the efficiency of a chromatographic column. It indicats the height of a theoretical plate in the column. The lower the HETP value, the more efficient the separation.
To calculate the HETP, the length of the column is divided by the number of theoretical plates (N) in the column. This provides the height equivalent to theoretical plate (HETP) value. The HETP value can be measured over time to monitor column performance.
The HETP value is an important parameter in chromatography as it determines the resolution of the separation. The resolution is the ability of the column to separate two or more components in a sample. A lower HETP value indicates a higher resolution and better separation.
The HETP value is a measure of the efficiency of a chromatographic column, calculated by dividing the length of the column by the number of theoretical plates. The lower the HETP value, the more efficient the separation and the better the resolution of the separation.
What Is the Unit of Measurement for HETP?
The unit for HETP (Height Equivalent to a Theoretical Plate) is typically expressed in units of length, such as meters or centimeters. HETP is a measure of the efficiency of a chromatography column, and represents the length of the column required for one theoretical plate to be formed. The smaller the HETP value, the more efficient the column, meaning that it can separate compounds more effectively. HETP values can be calculated using the equation HETP = L/N, whee L is the length of the column and N is the number of theoretical plates. It is important to note that HETP values can vary depending on the type of chromatography being used and the specific conditions of the experiment.
The Meaning of HETP in Distillation
HETP stands for Height Equivalent to a Theoretical Plate and is a commonly used term in the distillation industry to dscribe the performance of packed columns. It is a measure of the efficiency of a column in separating two or more components in a mixture.
In simple terms, HETP represents the height of packing material required to achieve the same degree of separation as one theoretical plate, which is a hypothetical unit that represents perfect separation. The smaller the HETP value, the more efficient the column is in separating the components.
HETP is influenced by several factors such as the type and size of the packing material, the flow rate of the liquid and vapor phases, and the physical properties of the components being separated.
In general, a lower HETP value is desirable as it indicates better separation efficiency and requires less packing material, which can result in cost savings. However, achieving a very low HETP value can be challenging and may require additional optimization of the column design and operating conditions.
To summarize, HETP is a measure of the efficiency of a packed distillation column and represents the height of packing material required to achieve the same degree of separation as one theoretical plate.
The Benefits of Using HETP
The height equivalent to a theoretical plate (HETP) is a commonly used term in chromatography design. It is used to measure the zone broadening of a solute peak in a chromatographic column. The concept of HETP is based on a theoretical model of column operation, but it is still used in practice because it provides a useful measure of the column’s performance.
HETP is calculated as the ratio of column length to the number of theoretical plates. A theoretical plate is a hypothetical separation stage in the column where the solute is equilibrated between the stationary and mobile phases. The lower the HETP value, the narrower is the solute peak, indicating better column performance.
HETP is used for several reasons, including:
1. Column design: HETP is used to design chromatographic columns with specific separation requirements. For example, if a narrow solute peak is required, a column with a low HETP value will be selected.
2. Column optimization: HETP is used to optimize chromatographic columns for improved performance. By measuring HETP, the column conditions can be adjusted to minimize zone broadening and improve separation efficiency.
3. Column comparison: HETP is used to compare the performance of different columns for a gven separation. Columns with lower HETP values are considered to be more efficient and may be preferred for certain applications.
HETP is a useful measure of column performance in chromatography design and optimization. It is used to design, optimize, and compare columns for improved separation efficiency.

Factors That Affect Hetp
HETP, or Height Equivalent to a Theoretical Plate, is an important parameter in chromatography that measures the efficiency of a chromatographic column. Several factors can affect the HETP value, including:
1. Temperature: The temperature of the chromatographic column can significantly affect the HETP value. As the temperature increases, the HETP decreases due to the increased diffusion of the tracer ions.
2. Flow rate: The HETP value is inversely proportional to the flow rate of the mobile phase. At higher flow rates, the HETP decreases, while at lower flow rates, the HETP increases.
3. Linear flow velocity: The HETP value is also influenced by the linear flow velocity. At higher linear flow velocities, the contribution from mass transfer limitations (c-term) increases, resulting in a higher back pressure.
4. Particle size: The HETP value is dependent on the particle size of the stationary phase. Smaller particles result in a lower HETP value due to the increased surface area avilable for analyte interaction.
5. Column length: The HETP value is directly proportional to the column length. Longer columns typically have a higher HETP value due to increased analyte interaction with the stationary phase.
6. Mobile phase composition: The HETP value can also be affected by the composition of the mobile phase. Changes in the pH, ionic strength, or organic modifier concentration can alter the analyte-stationary phase interaction, leading to changes in the HETP value.
The HETP value is influenced by a variety of factors, including temperature, flow rate, linear flow velocity, particle size, column length, and mobile phase composition. Understanding and controlling these factors can help optimize the efficiency of a chromatographic separation.
What is the Ideal Number of Theoretical Plates?
A good theoretical plate number is an important parameter to consider when performing chromatography. It is a measure of the efficiency of the chromatographic column and is calculated by dividing the column length by the number of theoretical plates. The theoretical plate number represents the number of equilibrium stages that the analyte must go through during the chromatographic process.
In general, a higher theoretical plate number indicates a more efficient column, which means bettr resolution and separation of analytes. A good theoretical plate number can range between 8000-12000, but this can vary depending on several factors. The flow rate of the mobile phase, the viscosity of the mobile phase, and the molecular weight of the compound being analyzed can all impact the theoretical plate number.
To achieve a good theoretical plate number, it is important to optimize the chromatographic conditions. This can involve adjusting the mobile phase composition, column temperature, and flow rate. It is also important to choose the appropriate stationary phase for the separation of the analytes of interest.
A good theoretical plate number is a key parameter to consider when performing chromatography. It represents the efficiency of the chromatographic column and can impact the resolution and separation of analytes. By optimizing chromatographic conditions and choosing the appropriate stationary phase, it is possible to achieve a good theoretical plate number and improve the quality of chromatographic separations.
The Benefits of Gas Absorption in HETP
In gas absorption, the HETP (Height Equivalent to a Theoretical Plate) is a crucial parameter that indicates the efficiency of the absorption process. It is the height of the packing required to provide the same level of separation as one theoretical plate. A theoretical plate is a hypothetical stage in a distillation column that provides perfect separation of the components in a mixture. In gas absorption, the HETP is used to measure the degree of mixing between the gas and liquid phases within a packed column.
The HETP is affected by several factors, including the physical properties of the gas and liquid phases, the flow rates of the gas and liquid, the type of packing material used, and the column diameter. The HETP is inversely proportional to the mass transfer coefficient, which is a measure of the rate at which the gas and liquid phases come into contact and exchange components.
To improve the efficiency of gas absorption, it is essential to minimize the HETP. This can be achieved by selecting the apropriate packing material and optimizing the flow rates of the gas and liquid phases. The HETP can also be reduced by increasing the column diameter, although this may not be practical in all cases due to space constraints.
The HETP is a critical parameter in gas absorption that indicates the efficiency of the process. It is the height of packing required to provide the same level of separation as one theoretical plate, and it is affected by several factors, including the physical properties of the gas and liquid phases, the flow rates of the gas and liquid, the type of packing material used, and the column diameter.
The Ideal Theoretical Plate
In numerous separation processes, an ideal theoretical plate is a hypothetical zone or stage where two phases, such as the liquid and vapor phases of a substance, reach equilibrium with each other. It is a theoretical concept that helps to explain the efficiency of separation processes.
An ideal theoretical plate is often considered as a single stage or zone within a separation column, which separates the components of a mixture. The concept of an ideal theoretical plate is crucial in the design and analysis of separation processes, such as distillation, absorption, and chromatography.
In a distillation column, for instance, the separation of two or more components of a mixture is achieved by repeatedly vaporizing and condensing the mixture. The vaporization and condensation occur on each theoretical plate, leading to a separation of the components based on their boiling points. The greater the number of theoretical plates, the more efficient the separation process will be.
An ideal theoretical plate is characterized by severl attributes, such as a uniform distribution of the phases, minimum mixing between the phases, and maximum contact time between the phases. In practical applications, however, ideal theoretical plates are difficult to achieve, and the actual separation efficiency may be lower than theoretical expectations.
An ideal theoretical plate is a theoretical concept used to explain the efficiency of separation processes. It is a crucial parameter in the design and analysis of separation processes, and the greater the number of theoretical plates, the more efficient the separation process will be.
Source: seeq.org
The Significance of a High Number of Theoretical Plates
In gas chromatography, theoretical plate number (N) is a measure of column efficiency. An increased number of theoretical plates means that a column is more efficient. Each theoretical plate represents an equilibrium stage in the separation process where the vapor mixture is distributed between the stationary and mobile phases.
A higher number of theoretical plates in a column leads to better separation because it allows more opportunities for the analyte to interact with the stationary phase and move thrugh the column. This results in sharper peaks in the chromatogram as the analytes are separated more effectively from one another.
In practical terms, the number of theoretical plates can be increased by using a longer column, smaller diameter packing particles, or a higher temperature. A high number of theoretical plates is important for achieving accurate and precise separations in analytical chemistry.
A high number of theoretical plates in gas chromatography indicates a more efficient column, which leads to better separation and sharper peaks in the chromatogram.
The Importance of Theoretical Plates
Theoretical plates are an essential aspect of column chromatography, which is a widely used technique in the separation and purification of chemical compounds. In simple terms, theoretical plates refer to a measure of the efficiency of a chromatography column, which is based on the number of times a solute interacts with the stationary phase and the mobile phase in the column.
The importance of theoretical plates in column chromatography lies in their direct relationship with the column’s efficiency. The more theoretical plates a column has, the better it can separate and purify chemical compounds from a mixture. This is because theoretical plates are directly proportional to the resolution of the column, which is the ability to separate two or more closely relaed compounds.
When all other variables are kept constant, the resolution of a column is directly proportional to the square root of the number of theoretical plates. Therefore, increasing the number of theoretical plates in a column can significantly improve its resolution and separation efficiency.
Here are some key points to summarize the importance of theoretical plates in column chromatography:
– Theoretical plates are a measure of the efficiency of a chromatography column.
– The more theoretical plates a column has, the better it can separate and purify chemical compounds from a mixture.
– Theoretical plates are directly proportional to the resolution of the column, which is the ability to separate two or more closely related compounds.
– Increasing the number of theoretical plates in a column can significantly improve its resolution and separation efficiency.
– Theoretical plates are essential for optimizing the performance of column chromatography and achieving high-quality separations.
The Impact of Flow Rate on HETP
When it comes to distillation processes, HETP (Height Equivalent to a Theoretical Plate) is a crucial parameter that determines the efficiency of the separation. HETP is defined as the height of packing material required to achieve the same degree of separation as one theoretical plate. The efficiency of the distillation column is directly proportional to the number of theoretical plates, which means that a smaller HETP value indicats a more efficient separation process.
Several factors can influence the HETP value, including temperature, pressure, and flow rate. In general, the higher the flow rate, the larger the HETP value. This is because a high flow rate can cause the liquid to flow too quickly through the column, reducing the time available for mass transfer to occur. As a result, the separation efficiency is reduced, and more plates are needed to achieve the same degree of separation.
Studies have shown that the relationship between HETP and flow rate is not linear. At low flow rates, HETP is relatively constant, but it starts to increase rapidly as the flow rate increases. This is because the liquid is moving too quickly through the column, leading to poor mass transfer and reduced separation efficiency.
It is worth noting that the effect of flow rate on HETP can vary depending on the type of column packing material used. For instance, structured packing tends to have a lower HETP value than random packing, even at high flow rates.
Flow rate can have a significant impact on the HETP value in distillation processes. To achieve optimal separation efficiency, it is essential to find the right balance between flow rate and other factors such as temperature and pressure.
Increasing Theoretical Plates in Distillation
Distillation is a widely used separation technique employed in various industries, including chemical, pharmaceutical, and petrochemical. The efficiency of distillation is oten measured by the number of theoretical plates, which represents the number of equilibrium stages required for complete separation of the components.
There are several ways to increase the number of theoretical plates in distillation, including:
1. Increasing the length of the column: As the length of the column increases, the number of theoretical plates also increases. This is because the longer the column, the greater the opportunity for the vapor and liquid phases to come into equilibrium.
2. Reducing the column diameter: A smaller column diameter increases the contact time between the vapor and liquid phases, resulting in more separation stages and higher number of theoretical plates.
3. Increasing reflux ratio: Reflux ratio is the ratio of liquid returned to the column to the amount of liquid removed as product. Increasing the reflux ratio increases the number of theoretical plates by increasing the liquid phase contact time and promoting more equilibration between the vapor and liquid phases.
4. Using structured packings: Structured packings increase the number of theoretical plates by providing a large surface area for the vapor and liquid phases to come into contact. This increases the contact time and promotes more equilibration between the phases.
5. Using optimal operating conditions: It is essential to operate the distillation column at optimal conditions, including temperature, pressure, and feed rate. Optimal operating conditions can help to maximize the number of theoretical plates by promoting the equilibrium between the phases.
There are several ways to increase the number of theoretical plates in distillation. The most straightforward approach is to increase the length of the column, but other options, such as reducing the column diameter, increasing the reflux ratio, using structured packings, and optimizing operating conditions, can also be beneficial.
Calculating Theoretical Plate Count
In chromatography, theoretical plate count is a critical parameter used to determine the performance and effectiveness of columns. It is an indirect measure of peak width for a peak at a specific retention time. The number of theoretical plates can be calculated using the following formula:
N = 16(tR / W)2
Where N is the theoretical plate count, tR is the retention time and W is the peak width.
To calculate the theoretical plate count, you need to determine the retention time of the analyte of interest and the peak width. The retention time is the time tken for the analyte to elute from the column, and the peak width is the width of the peak at its base.
Once you have determined these values, you can substitute them into the formula above to calculate the theoretical plate count for the column. It is important to note that the theoretical plate count is only an estimation and is affected by various factors such as the column material, particle size, and flow rate.
The formula for calculating theoretical plate count is N = 16(tR / W)2, where tR is the retention time and W is the peak width.
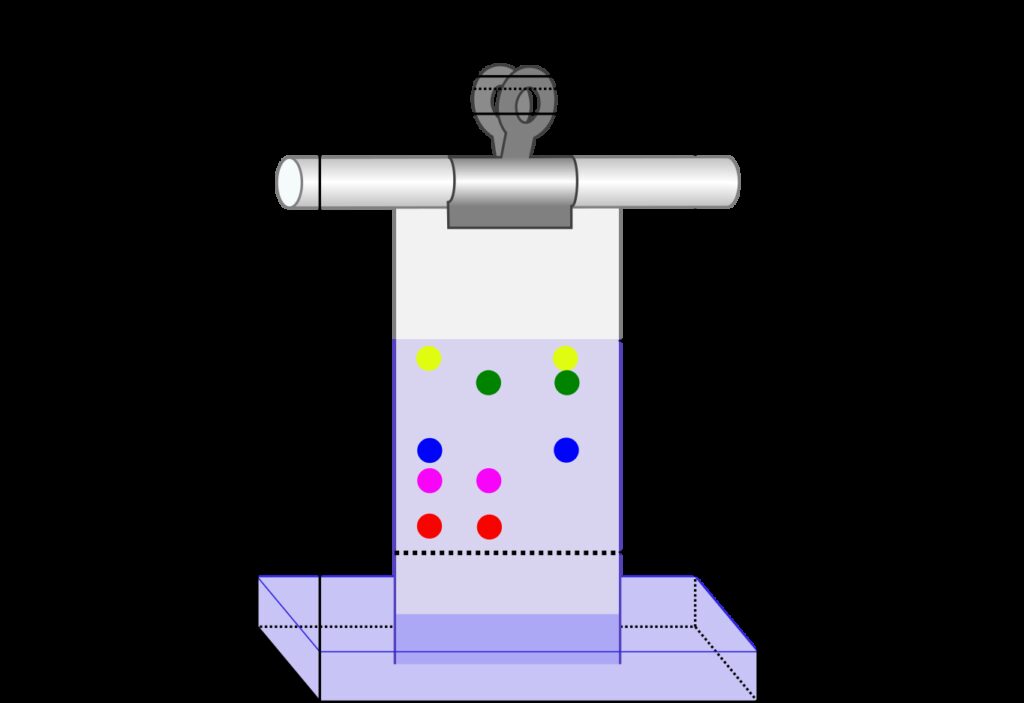
Theoretical Plates in HPLC
High-performance liquid chromatography (HPLC) is a widely used analytical technique in the field of chemistry for separating, identifying, and quantifying components in a mixture. The efficiency of the HPLC column is a critical factor that determines the resolution and accuracy of the separation process. The theoretical plate is a measure of the column’s efficiency and is defined as a hypothetical zone or stage in which the stationary phase and the liquid mobile phase establish an equilibrium with each other.
In HPLC, the stationary phase is typically a solid material that is packed into a column, while the mobile phase is a liquid that flows through the column. As the sample moves through the column, it interacts with the stationary phase, leading to separation of the components based on their chemical properties. The efficiency of the separation process is determined by the number of theoretical plates in the column.
A theoretical plate is a hypothetical zone in which the sample equilibrates with the stationary phase. The higher the number of theoretical plates in a column, the better the separation efficiency. The number of theoretical plates in a column can be calculated usig the following formula:
N = (5.54 * (tR/w)^2)
Where N is the number of theoretical plates, tR is the retention time of the solute, and w is the peak width at half height.
Theoretical plates can be improved by optimizing the column packing, selecting the appropriate stationary phase, and adjusting the mobile phase composition. In general, longer columns with smaller particle sizes and increased pressure can lead to higher numbers of theoretical plates and better separation efficiency.
Theoretical plates are a measure of the efficiency of an HPLC column in separating components in a mixture. The higher the number of theoretical plates, the better the separation efficiency. The number of theoretical plates can be calculated using a formula that takes into account the retention time of the solute and the peak width at half height. Optimization of the column packing, stationary phase, and mobile phase can improve the number of theoretical plates in an HPLC column.
Conclusion
The height equivalent to a theoretical plate (HETP) is a crucial parameter in chromatography and other separation processes. It is a measure of the efficiency of a column, indicating the distance that a molecule must travel to achieve a separation equivalent to that of one theoretical plate. The lower the HETP value, the more efficient the separation. By monitoring the HETP value over time, column performance can be evaluated and optimized.
Understanding HETP is essential for achieving high-quality separations and maximizing the efficiency of separation processes. With the right column and operating conditions, it is possible to achieve low HETP values and theefore achieve high-quality separations. Therefore, it is important to carefully consider the HETP value when selecting a column and optimizing separation conditions.
