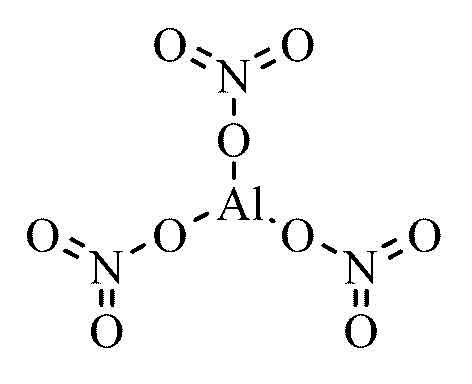
Aluminum nitrate is a chemical compound that is commonly used in various industrial processes. Its molecular formula is Al(NO3)3, which means it is made up of one aluminum atom and three nitrate ions. This compound is very important in various fields such as agriculture, pharmaceuticals, and chemical manufacturing.
The chemical formula for aluminum nitrate is derived from the combination of two chemical species: aluminum and nitrate. Aluminum is a metal that is found in abundance on Earth and is known for its excellent conductivity and light weight. Nitrate is an ion that is formed by the combination of nitrogen and oxygen atoms. Nitrate is commonly found in fertilizers and is used to enhance plant growth.
When aluminum and nitrate combine, they form a compound with a net charge of zero. The aluminum ion has a charge of +3, while the nitrate ion has a charge of -1. To balance the charges, three nitrate ions are needed for every one aluminum ion, resulting in the formula Al(NO3)3.
In one formula unit of Al(NO3)3, there would be nine atoms of oxygen. In Al(NO3)3, there are three nitrate ions (as shown by the three on the outide of the parenthesis), each of which have three oxygen atoms. This means that there are nine oxygen atoms in total in one formula unit of aluminum nitrate.
Aluminum nitrate is a white crystalline substance that is soluble in water. It is commonly used as a coagulant in water treatment plants to remove impurities from drinking water. It is also used in the production of other chemicals such as aluminum oxide, which is used in the production of aluminum metal.
The chemical formula for aluminum nitrate is Al(NO3)3, which is derived from the combination of aluminum and nitrate ions. It has numerous industrial applications and is an important compound in various fields.
The Formula for Aluminium Nitrate
The chemical formula for aluminium nitrate is Al(NO3)3. This formula represents the chemical composition of the compound, which is made up of one aluminium ion (Al3+) and three nitrate ions (NO3-). The aluminium ion has a positive charge of three, whle each nitrate ion has a negative charge of one. The combination of these ions results in a neutral compound.
Aluminium nitrate is commonly used as a reagent in various chemical reactions, such as in the production of other aluminium compounds and in the manufacturing of dyes and pigments. It is also used as a mordant in textile dyeing and printing processes.
The formula for aluminium nitrate is important because it provides information about the chemical structure of the compound. This information is crucial for understanding the properties and behavior of the compound in different chemical reactions. For example, the fact that aluminium nitrate contains three nitrate ions indicates that it has a high solubility in water, which is important for its use in various industries.
The formula for aluminium nitrate is Al(NO3)3, which represents the chemical composition of the compound. This formula is important for understanding the properties and behavior of the compound in different chemical reactions and for its use in various industries.
Source: aluminummanufacturers.org
Is Al(NO3)3 a Formula Unit?
Al(NO3)3 is a formula unit. A formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. In the case of Al(NO3)3, it is a chemical compound that consists of one aluminum cation (Al3+) and three nitrate anions (NO3−). The formula unit of Al(NO3)3 represents the ratio of atoms or ions in the compound, indicating that for every one aluminum ion, there are three nitrate ions. This ratio is essential in determining the stoichiometry of chemical reactions. It is also important to note that the formula unit of Al(NO3)3 contans a total of nine atoms of oxygen, which are essential for the formation of the nitrate anions. the formula unit of Al(NO3)3 is crucial in understanding the chemical and physical properties of the compound.
Some key points to summarize:
– Al(NO3)3 is a formula unit
– Formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations
– Al(NO3)3 consists of one aluminum cation (Al3+) and three nitrate anions (NO3−)
– The formula unit of Al(NO3)3 represents the ratio of atoms or ions in the compound, indicating that for every one aluminum ion, there are three nitrate ions
– The formula unit of Al(NO3)3 contains a total of nine atoms of oxygen, which are essential for the formation of the nitrate anions.
The Chemical Composition of Al2 NO3 3
Al2(NO3)3, also known as aluminum nitrate, is a chemical compound composed of aluminum cations (Al3+) and nitrate anions (NO3-). It is a white crystalline solid and is soluble in water. The formula for aluminum nitrate indicates that it contains two aluminum ions and three nitrate ions.
Aluminum nitrate is commonly used in the production of aluminum oxide, which is used as a catalyst in various chemical reactions. It is also used in the manufacturing of other aluminum compounds, such as aluminum hydroxide and aluminum sulfate.
In addition, aluminum nitrate is used in the production of fertilizers, particularly those that are rich in nitrogen. It is also used in the manufacture of flame-retardant materials, as well as in the production of dyes, pigments, and other chemicals.
It is important to note that aluminum nitrate can be hazardous if not handled properly. It can caue skin and eye irritation, as well as respiratory problems if inhaled. It is recommended that protective equipment, such as gloves and goggles, be worn when handling this compound.
Is Aluminum Nitrate Trihydrate Aqueous or Solid?
Aluminum nitrate, denoted by the chemical formula Al(NO3)3, is a solid compound according to the Standard Temperature and Pressure (STP) conditions. At STP, which is defined as a temperature of 0 °C and a pressure of 1 atm, aluminum nitrate exists as a crystalline solid that is white or colorless in appearance.
It is important to note that aluminum nitrate can also exist in an aqueous state, meaning that it is dissolved in water. When aluminum nitrate is dissolved in water, it dissociates into its constituent ions, which are Al3+ and NO3-. This results in the formation of an aqueous solution of aluminum nitrate.
However, the original question specifically asks whethr aluminum nitrate is aqueous or solid, and under STP conditions, it is a solid. It should also be noted that the physical state of aluminum nitrate can change depending on the temperature and pressure conditions it is subjected to.
Aluminum nitrate is a solid compound under STP conditions, but it can also exist in an aqueous state when dissolved in water.
The Chemical Composition of Al HNO3
Al HNO3 is not a specific compound or substance. It is a combination of two chemicals, aluminum and nitric acid. When aluminum reacts with nitric acid, the resulting chemical reaction produces aluminum nitrate and hydrogen gas.
The chemical formula for this reaction is:
Al + HNO3 → Al(NO3)3 + H2
In this reaction, the aluminum (Al) reacts with nitric acid (HNO3) to form aluminum nitrate (Al(NO3)3) and hydrogen gas (H2). Aluminum nitrate is a salt that is commonly used in the production of ceramics, in the textile industry, and as a component in fertilizer.
It is important to note that this reaction should be carried out with caution as it produces hydrogen gas, which is highly flammable and can be dangerous if not handled properly. Additionally, nitric acid is a strong acid that can be corrosive and potentially harmful if it comes into contact with skin or eyes.
Al HNO3 is a chemical reaction that results in the formation of aluminum nitrate and hydrogen gas. It is a usefl reaction in various industries but should be conducted with care and proper safety measures in place.

Is Aluminum Nitrate an Acid?
Al(NO3)3 is a chemical compound that consists of one aluminum ion and three nitrate ions. To determine whether Al(NO3)3 is an acid or a base, we need to look at the properties of its constituent ions.
Aluminum ions (Al3+) have a relatively low electronegativity, meaning they tend to donate electrons in chemical reactions. This property makes aluminum ions more likely to act as Lewis acids, which are compounds that accept electron pairs from other compounds. However, aluminum ions also have a strong affinity for hydroxide ions (OH-) due to their charge, so they can also act as weak bases.
Nitrate ions (NO3-) are polyatomic ions that consist of one nitrogen atom and three oxygen atoms. Nitrate ions are typically stable and do not donate or accept electrons in chemical reactions. However, nitrate ions can form strong acids when combined with hydrogen ions (H+).
When aluminum nitrate (Al(NO3)3) is dissolved in water, it dissociates into aluminum ions and nitrate ions. Since nitrate ions can form strong acids, this means that Al(NO3)3 is an acidic compound. However, the acidity of Al(NO3)3 is relatively weak compared to strong acids like hydrochloric acid (HCl) or sulfuric acid (H2SO4).
Al(NO3)3 is an acidic compound due to the presence of nitrate ions, which can form strong acids. However, the acidity of Al(NO3)3 is relatively weak compared to other strong acids.
The Ionization State of Aluminum
The correct notation for the aluminum cation is Al3+. The superscript 3+ indicates that the aluminum ion has lost three electrons, giving it a positive charge of three. The space between the “Al” and the “3+” is not necessary and is often omitted. It is important to note that the aluminum cation is a common ion in many chemical reactions and is important in various industries such as construction, automotive, and aerospace.
Uses of Al2O3
Aluminium oxide, also known as alumina, is a widely used compound due to its numerous beneficial properties. It is a white crystalline powder that is chemically stable and has high thermal conductivity. Here are some of the main reasons why Al2O3 is used:
1. Water purification: Al2O3 is an effective adsorbent and is commonly used in water purification processes. It is capable of removing impurities such as fluoride, arsenic, and lead from water, making it safe for consumption.
2. Abrasive: Due to its hardness and strength, Al2O3 is used as an abrasive in vrious applications, such as in sandpaper, cutting tools, and grinding wheels. It can also be used as a polishing agent for metals and glass.
3. Electrical insulator: Al2O3 is an excellent electrical insulator and is commonly used as a substrate for integrated circuits. It is also used as a dielectric material in capacitors and other electronic components.
4. Refractory material: Al2O3 has a high melting point and is resistant to heat, making it an ideal material for refractory applications. It is used in the production of ceramics, glass, and cement.
5. Catalyst: Al2O3 is used as a catalyst in various chemical reactions, such as in the production of ammonia and petroleum refining. It is also used as a support material for catalysts in catalytic converters.
Al2O3 is a versatile compound that finds application in numerous industries due to its unique properties. Its use in water purification, abrasives, electrical insulation, refractory materials, and catalysts makes it an essential compound in modern technology.
Conclusion
Aluminum nitrate is a chemical compound with the formula Al(NO3)3. This compound is commonly used in varius industrial processes such as the production of aluminum alloys, in the manufacturing of ceramics, and as a chemical reagent in the laboratory.
The chemical formula for aluminum nitrate reveals that it contains one aluminum ion (Al3+) and three nitrate ions (NO3-). Each nitrate ion contains one nitrogen atom and three oxygen atoms, making a total of nine oxygen atoms in one formula unit of Al(NO3)3.
The molar mass of aluminum nitrate is 213.00 g/mol, and its density is 1.72 g/cm³. This compound is soluble in water and has a white crystalline appearance.
Aluminum nitrate is a strong oxidizing agent that can react with flammable materials and cause fires or explosions. It is also considered to be harmful if ingested or inhaled, and can cause skin and eye irritation upon contact.
The formula for aluminum nitrate is Al(NO3)3, which contains one aluminum ion and three nitrate ions. This compound has various industrial applications but should be handled with caution due to its oxidizing and harmful properties.
