Pigments are a class of molecules that play a crucial role in absorbing light in various biological and chemical processes. These molecules absorb specific wavelengths of light and reflect or transmit the rest of the spectrum, giving them their characteristic colors. But how do pigments absorb light, and what happens to them once they do?
At a molecular level, pigments absorb light by a process called excitation. This process involves the absorption of a photon of light by the pigment molecule, which causes one of its electrons to jump from its ground state to an excited state. In oter words, the electron is promoted to a higher energy level, further from the nucleus.
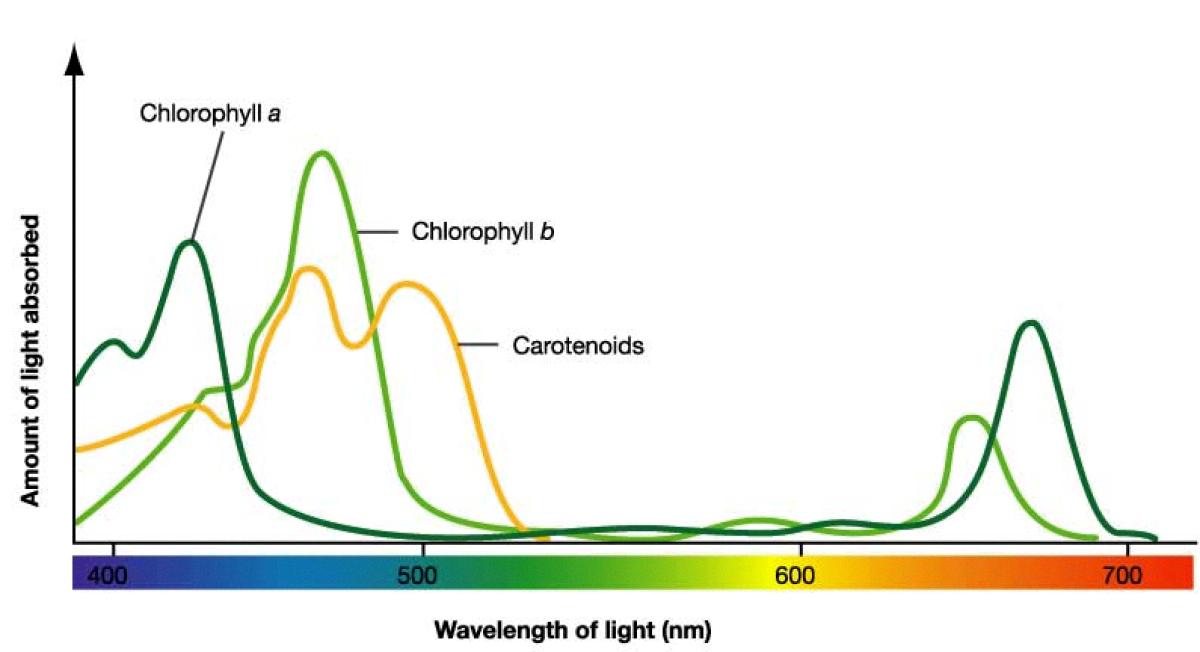
The amount of energy required to excite an electron depends on the pigment’s molecular structure, and it varies depending on the pigment’s color. For example, chlorophyll, the pigment responsible for green color in plants, absorbs light in the red and blue regions of the spectrum but reflects green light, giving leaves their characteristic color.
Once a pigment absorbs a photon of light, it becomes excited and unstable, and it can return to its ground state by releasing the absorbed energy in several ways. One way is by emitting light at a slightly lower energy level than the absorbed photon, a process known as fluorescence. This fluorescence event transfers the absorbed energy to neighboring pigments, which then becomes excited and transfer the energy further in a process called resonance energy transfer.
This transfer of excitation energy from one pigment to another is called the antenna effect, and it is crucial in photosynthesis. The antenna effect ensures that sufficient energy is transferred to the reaction center, where it is used to drive chemical reactions that convert light energy into chemical energy.
Pigments absorb light by a process called excitation, which involves the promotion of an electron to a higher energy level. Once excited, the pigment releases the absorbed energy through fluorescence and resonance energy transfer, which eventually leads to the reaction center. Understanding how pigments absorb light is crucial in many fields, including biochemistry, material science, and optics, and it has many practical applications, including the development of new photovoltaic materials and light-harvesting technologies.
The Effects of Light on Pigments
When pigments absorb a photon of light, it undergoes a process called excitation. This means that the pigment molecule gains extra energy and is no longer in its ground state. At a subatomic level, excitation occurs when an electron within the pigment molecule is bumped into a higher-energy orbital that lies frther from the nucleus. This movement of electrons is what creates the excited state of the pigment. Once the pigment is excited, it can then undergo various chemical reactions that allow it to transfer its energy to other molecules or to produce a biological response, such as the triggering of a neural signal in the case of vision. The specific wavelength of light that a pigment absorbs depends on its molecular structure, and different pigments will absorb different colors of light. Overall, the absorption of light by pigments is a key process in many biological systems, allowing organisms to sense and respond to their environment.
Source: ressources.unisciel.fr
How Pigments Capture Light
Pigments capture light by absorbing photons of a specific wavelength. When a photon strikes a pigment molecule, it excites one of its electrons to a higher energy level. This excited state is unstable and quickly dissipates, resulting in the release of the absorbed energy as heat or fluorescence, or it can be passed to other nearby pigments. This process, known as resonance energy transfer, continues until the energy reaches the reaction center, whee it triggers a chemical reaction. The specific wavelengths of light that a pigment can absorb depend on its molecular structure and the arrangement of its electrons. The absorption spectrum of a pigment shows the range of wavelengths it can absorb, and this can be used to identify the pigment and study its function.
Do Pigments Absorb Photons?
Yes, pigments do absorb some photons. Pigments are molecules that have the ability to absorb certain wavelengths of light and reflect others, giving them their characteristic color. When a photon of light strikes a pigment molecule, it can be absorbed by the molecule’s electrons, causing them to become excited and enter a higher energy state. This energy can then be transferred to neighboring pigment molecules through a process called fluorescence, where the excited electron emits a photon of light and returns to its ground state. This transfer of energy continues untl it reaches the reaction center, where it is used to transfer an energetic electron to an electron acceptor, initiating the process of photosynthesis. Therefore, the absorption of photons by pigments is a critical step in the conversion of light energy into chemical energy in photosynthesis.

Conclusion
In conclusion, pigments do absorb light by capturing photons of specific wavelengths. This absorption process excites the pigment at a subatomic level, causing one of its electrons to jump to a higher energy level. The excitation is then transmitted though a network of pigments until it reaches the reaction center, where the energy is utilized in various ways, such as transferring an electron to an electron acceptor. This process is crucial in photosynthesis and enables plants and other organisms to convert light energy into chemical energy that can be used to fuel their biological processes. Understanding how pigments absorb light is essential in various fields, including biochemistry, biophysics, and plant physiology, and has significant implications for the development of new technologies in renewable energy and agriculture.
