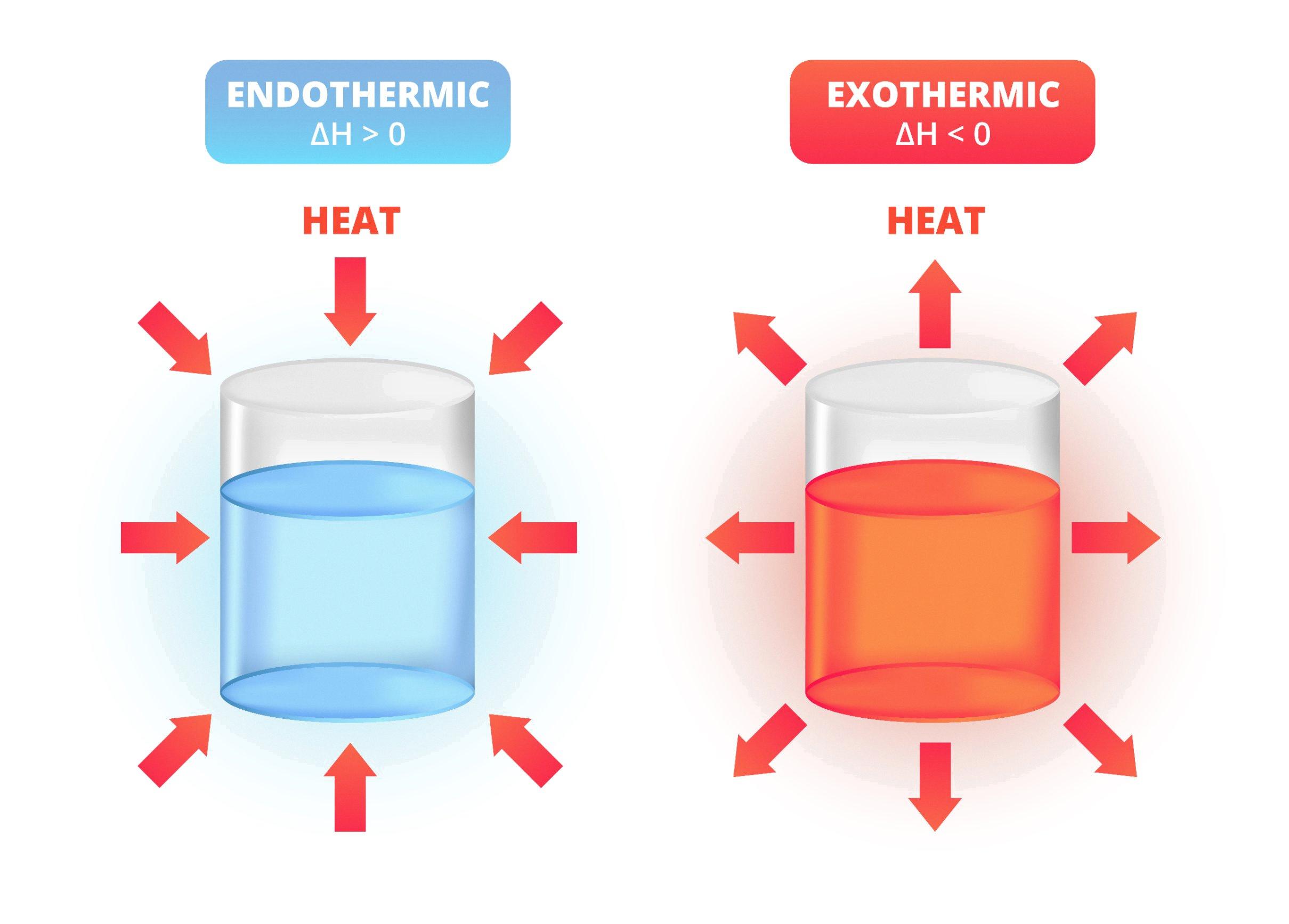
Endothermic reactions are a fascinating topic in chemistry. In these reactions, the system absorbs heat from its surroundings, resulting in a decrease in temperature of the surroundings. But do endothermic reactions feel cold? Let’s explore this concept in detail.
First, it’s essential to understand what an endothermic reaction is. In simple terms, an endothermic reaction is a chemical reaction that requires energy input to proceed. In these reactions, the energy is absorbed from the surroundings, causing a temperature drop. Some common examples of endothermic reactions include melting of ice, evaporation of water, and photosynthesis.
Now, coming to the question of wheter endothermic reactions feel cold or not, the answer is yes. When an endothermic reaction occurs, heat is absorbed from the surroundings, leading to a decrease in temperature. As a result, the surroundings feel cold. This is why melting ice feels cold to the touch, and a cold pack becomes cold when activated.
It’s important to note that not all reactions that feel cold are endothermic. For example, when we touch a metal surface in winter, it feels cold to the touch. However, this is not due to an endothermic reaction but rather because metals are excellent conductors of heat, and they draw heat away from our skin.
In contrast, exothermic reactions release heat to the surroundings, causing an increase in temperature. Burning wood in a fireplace is an example of an exothermic reaction. These reactions feel hot because they release heat into the surroundings.
Endothermic reactions do feel cold because they absorb heat from their surroundings, causing a temperature drop. These reactions are essential in many natural and industrial processes, and understanding how they work is vital in the field of chemistry.
Is Feeling Cold an Exothermic or Endothermic Process?
Feeling cold is an endothermic process. When your body feels cold, it means that heat is being transferred from your body to the surroundings. This is because your body is warmer than the surrounding environment, and heat naturally flows from warmer objects to cooler ones. As your body loses heat to the surroundings, it cools down and you feel cold. This transfer of heat from your body to the surroundings is an endothermic process because it requires an input of energy in the form of heat from the surroundings. Therefore, feeling cold is an example of an endothermic process.
Source: online-learning-college.com
Do Exothermic Reactions Feel Cold?
No, exothermic reactions do not feel cold. In fact, exothermic reactions usually feel hot because they release energy in the form of heat. The term “exothermic” itself means “outside heat,” indicating that heat is being released from the reaction. It is important to note that some exothermic reactions may not feel hot to the touch, but they still release heat that can be measured with thermometers or other instruments. However, it is possible to have a reaction that feels cold to the touch, whch is known as an endothermic reaction. In these reactions, energy is absorbed from the surroundings, resulting in a decrease in temperature.
The Effects of Endothermic Heating and Cooling
Endothermic reactions are associated with cooling. This is because endothermic reactions absorb heat from their surroundings, which results in a decrease in temperature. For example, when ice melts, it absorbs heat from the surrounding environment, resulting in a decrease in temperature. Similarly, when a hand warmer is activated, the chemicals inside the hand warmer undergo an endothermic reaction, absorbing heat from the surrounding environment and producing a cooling effect. Therefore, endothermic reactions are typically associated with cooling rather than heating.
Are Endothermic Reactions Warm to the Touch?
No, endothermic reactions are not warm to touch. In fact, they feel cold to the touch as they absorb heat from the surroundings. This is bcause endothermic reactions require energy input to proceed, and they absorb thermal energy from the environment to facilitate the reaction. As a result, the temperature of the surroundings decreases, and the reaction feels cold to the touch. It is important to note that not all cold reactions are necessarily endothermic, and not all warm reactions are exothermic. The temperature change observed will depend on the specific reaction and the conditions under which it takes place.
Is Freezing an Exothermic Process?
Freezing is a process where a substance changes its state from liquid to solid due to a decrease in temperature. In most cases, freezing is an exothermic process, which means that heat is released durng the transformation. This is because when a liquid substance is cooled, its molecules lose energy, and they start to move slower and closer together. As a result, the attractive forces between the molecules become stronger, which leads to the formation of a more organized and stable solid structure.
However, there are some exceptions where freezing can be endothermic, meaning that heat is absorbed during the process. One example is the freezing of certain solutions that contain solutes, such as saltwater. In such cases, the solute particles can interfere with the formation of the solid structure, and more energy is required to overcome these obstacles, resulting in an endothermic process.
Overall, while freezing is mostly exothermic, there are some cases where it can be an endothermic process depending on the properties of the substance being frozen.

The Coldest Endothermic Reaction
The coldest endothermic reaction ever recorded was performed by cooling molecules to just above absolute zero. Absolute zero is the lowest possible temperature, which is -273.15° C or -459.67° F. During this experiment, two molecules were observed swapping atoms, a phenomenon that had never been seen before. This groundbreaking research could have significant implications for our understanding of chemical reactions and the behavior of matter at extremely low temperatures.
Does Endothermic Reaction Increase Temperature?
No, endothermic reactions do not increase temperature. In fact, they have the opposite effect. Endothermic reactions absorb energy from the surroundings, usually in the form of heat, and convert it into chemical energy. This means that the temperature of the surroundings decreases as energy is absorbed. For example, when ice melts, it undergoes an endothermic reaction where heat is absorbed from the surroundings to change the solid ice into liquid water. As a result, the temperature of the surroundings decreases. So, endothermic reactions result in a decrease in temperature rather than an increase.
The Effects of Exothermic Reactions on Temperature
Exothermic reactions release energy as they proceed, and this energy is typically in the form of heat. When the bonds are formed in the products, more energy is released than is required to break the bonds in the reactants. This means that the excess energy is released as heat, which can increase the temperature of the surrounding environment.
For example, when a fire burns, it is an exothermic reaction that releases energy in the form of heat and light. As the fuel (reactant) burns, it releases energy that is used to break the bonds in the fuel molecules and form new bonds with oxygen molecules from the air (product). The excess energy is released as heat, which can cause the temperature of the surrounding air to rise.
Similarly, many chemical reactions that occur in our bodies are exothermic, such as the breakdown of glucose during cellular respiration. This releases energy that is used by our cells to perform various functions. However, if too much energy is released too quickly, it can cause our body temperature to rise, leading to heat exhaustion or even heat stroke.
Overall, exothermic reactions get hotter becase they release energy in the form of heat, which increases the temperature of the surrounding environment.
The Difference Between Exothermic and Endothermic Reactions
Exothermic and endothermic reactions are two types of chemical reactions that differ in the way they handle energy. The main difference between the two is that exothermic reactions release energy to the surroundings, while endothermic reactions absorb energy in the form of heat from their surroundings. In an exothermic reaction, energy is released as the reactants are converted into products, resulting in a temperature increase in the surroundings. Conversely, in an endothermic reaction, energy is absorbed from the surroundings, which cases a decrease in temperature. It is important to note that the energy change in a reaction can be measured in terms of enthalpy, which is the heat content of a system at constant pressure.

Is Water Cooling Endothermic?
Water cooling is an endothermic process, which means that it absorbs heat energy from its surroundings. This is because, during the cooling process, heat energy is transferred from the object beig cooled to the water, causing the water to increase in temperature. As a result, the water molecules become more energetic, and the temperature of the water rises. This phenomenon is commonly observed in many cooling systems, such as cooling towers, air conditioning units, and refrigeration systems. In summary, water cooling is an endothermic process that absorbs heat energy from its surroundings to reduce the temperature of the object being cooled.
Conclusion
To conclude, endothermic reactions are a type of chemical reaction that involves the absorption of heat from their surroundings. These reactions are important in many natural and industrial processes, including melting, evaporation, and photosynthesis. Endothermic reactions have a positive change in enthalpy, and they result in a decrease in the temperature of the surroundings. These reactions can be used to cool down a system or to absorb excess energy from a chemical reaction. Understanding the principles of endothermic reactions is essential for scientists, engineers, and anyone working with chemical processes. By studying the properties and behavior of endothermic reactions, we can develop new technologies and processes that are more efficient and sustainable. Therefore, endothermic reactions play a crucial role in the field of chemistry and have many practical applications in our daily lives.
