Molecular geometry is an important concept in the field of chemistry as it helps us understand the three-dimensional arrangement of atoms in a molecule. A molecule is said to be planar when all of its atoms lie in the same plane, while a non-planar molecule has atoms that are not all in the same plane. In this article, we will explore the concept of non-planar molecules and discuss which of the following compounds is not planar: Al2Cl6, diborane (B2H6), BF3, NF3, and XeF4.
Let’s start by analyzing Al2Cl6. This compound consists of two aluminum atoms bonded to six chlorine atoms. If we draw the Lewis structure for Al2Cl6, we can see that the aluminum atoms are surrounded by six chlorine atoms, resulting in a trigonal planar arrangement. However, when we consider the shape of the molecule in three dimensions, we find that the chlorine atoms are not all in the same plane as the aluminum atoms. Therefore, Al2Cl6 is a non-planar molecule.
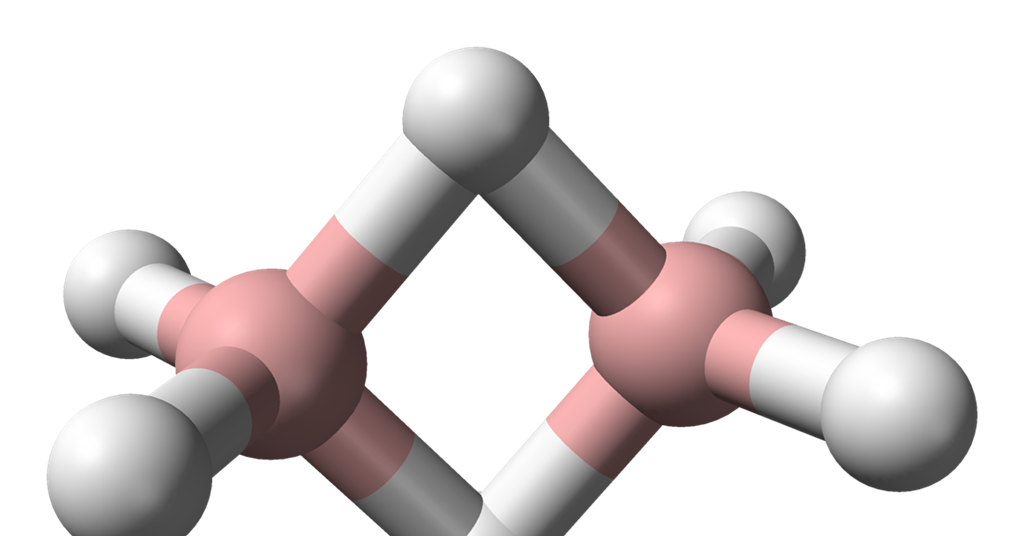
Moving on to diborane (B2H6), this compound is made up of two boron atoms bonded to six hydrogen atoms. The Lewis structure of diborane shows that each boron atom is bonded to three hydrogen atoms. If we consider the three-dimensional arrangement of the atoms, we find that the hydrogen atoms are not all in the same plane as the boron atoms. Instead, they are present above and below the plane of the boron-hydrogen bond. Therefore, diborane is a non-planar molecule.
Now, let’s examine BF3. This compound consists of one boron atom bonded to three fluorine atoms. The Lewis structure of BF3 reveals that boron has no lone pairs of electrons and is surrounded by three fluorine atoms in a trigonal planar arrangement. The three fluorine atoms are all in the same plane as the boron atom, making BF3 a planar molecule.
Next, we have NF3. This compound is similar to BF3 in terms of its structure, with one nitrogen atom bonded to three fluorine atoms. However, the key difference lies in the lone pair of electrons present on the nitrogen atom in NF3. This lone pair of electrons disrupts the symmetry of the molecule, causing the fluorine atoms to be in a pyramidal arrangement rather than in the same plane as the nitrogen atom. Therefore, NF3 is a non-planar molecule.
Lastly, let’s consider XeF4. This compound consists of one xenon atom bonded to four fluorine atoms. The Lewis structure of XeF4 shows that xenon has two lone pairs of electrons and is surrounded by four fluorine atoms in a square planar arrangement. All the atoms and lone pairs lie in the same plane, making XeF4 a planar molecule.
Out of the compounds mentioned, Al2Cl6, diborane, and NF3 are non-planar molecules, while BF3 and XeF4 are planar molecules. Understanding the concept of molecular geometry and the arrangement of atoms in three dimensions is crucial in predicting the properties and behavior of molecules.
Which Of The Following Is Non Planar?
Diborane (B2H6) is a molecule that exhibits a non-planar structure. This means that the atoms within the molecule are not all located in the same plane. In the case of diborane, there are two hydrogen atoms that are positioned above and below the plane formed by the boron and hydrogen atoms. This non-planarity is a result of the electron configuration and bonding in the molecule.
The non-planar structure of diborane can be visualized as follows:
– The central boron atom is surrounded by four hydrogen atoms, forming a tetrahedral arrangement.
– However, there are two additional hydrogen atoms that are attached to each boron atom. These additional hydrogen atoms are located above and below the plane formed by the central boron and hydrogen atoms.
– This arrangement results in a three-dimensional structure, with the two hydrogen atoms above the plane and the two hydrogen atoms below the plane.
The non-planar structure of diborane is due to the presence of empty p-orbitals on the boron atoms, which allow for the formation of three-center two-electron bonds. This type of bonding leads to the distortion of the molecule from a perfect tetrahedral geometry to a non-planar structure.
Diborane is a molecule that has a non-planar structure, with two hydrogen atoms positioned above and below the plane formed by the boron and hydrogen atoms.
Is BF3 A Non Planar?
BF3 is actually a planar molecule. The central atom in BF3 is boron, which is surrounded by three fluorine atoms. The boron atom does not have any lone pairs of electrons. This arrangement leads to a trigonal planar geometry, where all the atoms lie in the same plane.
The reason BF3 is planar is because the boron atom forms three sigma bonds with the fluorine atoms, resulting in a trigonal planar shape. The bond angles between the boron and fluorine atoms are approximately 120 degrees.
In contrast, if we compare NF3, the central atom is nitrogen, which has one lone pair of electrons. This lone pair of electrons affects the geometry of the molecule, causing NF3 to adopt a pyramidal shape. The lone pair of electrons repels the bonding pairs, resulting in a slight distortion from the trigonal planar geometry.
BF3 is a planar molecule due to the absence of any lone pairs of electrons on the central boron atom.
Conclusion
The molecules Al2Cl6, diborane, and NF3 are all examples of non-planar structures. Al2Cl6 adopts a non-planar geometry due to the presence of two chlorine atoms above and below the plane of the aluminum atoms. Similarly, diborane exhibits a non-planar structure with two hydrogen atoms positioned above and below the plane of the boron atoms. On the other hand, BF3 and XeF4 are both planar molecules. BF3 lacks a lone pair of electrons, resulting in a flat, trigonal planar geometry. XeF4, despite having four fluorine atoms, also adopts a planar arrangement due to the presence of two lone pairs of electrons on the central xenon atom. Understanding the geometries and structures of these molecules is crucial in predicting their chemical properties and behavior.
