In the vast realm of organic chemistry, hydrocarbons play a fundamental role. They are compounds composed solely of carbon and hydrogen atoms, and serve as the building blocks for many organic substances found in nature. Hydrocarbons can be classified into two main categories based on the presence or absence of double or triple bonds: saturated and unsaturated hydrocarbons.
Saturated hydrocarbons, also known as alkanes, are characterized by single covalent bonds between carbon atoms, resulting in a fully saturated carbon chain. These compounds are relatively stable and less reactive compared to their unsaturated counterparts. Alkanes have a general formula of CnH2n+2, where ‘n’ represents the number of carbon atoms in the chain.
On the other hand, unsaturated hydrocarbons contain double or triple bonds between carbon atoms. These multiple bonds introduce a level of reactivity and chemical diversity that distinguishes them from saturated hydrocarbons. Unsaturated hydrocarbons can be further classified into two groups: alkenes and alkynes.
Alkenes are unsaturated hydrocarbons that contain at least one carbon-carbon double bond. They have the general formula of CnH2n, where ‘n’ represents the number of carbon atoms in the chain. The presence of a double bond introduces a degree of unsaturation and reduces the number of hydrogen atoms in the molecule when compared to an alkane with the same number of carbon atoms.
For example, let’s consider ethene, a simple alkene with two carbon atoms. Its molecular formula is C2H4. It has a double bond between the two carbon atoms, resulting in four hydrogen atoms bonded to each carbon atom. This conforms to the general formula CnH2n.
Similarly, alkynes are unsaturated hydrocarbons that contain at least one carbon-carbon triple bond. Their general formula is CnH2n-2, where ‘n’ represents the number of carbon atoms in the chain. Alkynes have even fewer hydrogen atoms compared to alkenes with the same number of carbon atoms.
To summarize, unsaturated hydrocarbons are characterized by the presence of double or triple bonds, which contribute to their reactivity and chemical diversity. Alkenes, with a double bond, have the general formula CnH2n, while alkynes, with a triple bond, have the general formula CnH2n-2. Understanding the formulas of unsaturated hydrocarbons is crucial in grasping their structural and chemical properties.
What Is The Unsaturated Hydrocarbon Formula?
The unsaturated hydrocarbon formula is CnH2n – 2. This formula is applicable to compounds that contain double or triple bonds, which are collectively referred to as unsaturated compounds. Unlike alkanes and aromatic hydrocarbons, alkenes and alkynes, which are examples of unsaturated hydrocarbons, have multiple bonds and are generally more chemically reactive. Here are some key points about unsaturated hydrocarbons:
– Unsaturated hydrocarbons have a general formula of CnH2n – 2.
– They are compounds that contain double or triple bonds.
– Alkenes and alkynes are examples of unsaturated hydrocarbons.
– Unsaturated hydrocarbons are more chemically reactive compared to alkanes and aromatic hydrocarbons.
The formula for unsaturated hydrocarbons is CnH2n – 2, and they are characterized by their multiple bonds and higher reactivity compared to alkanes and aromatic hydrocarbons.
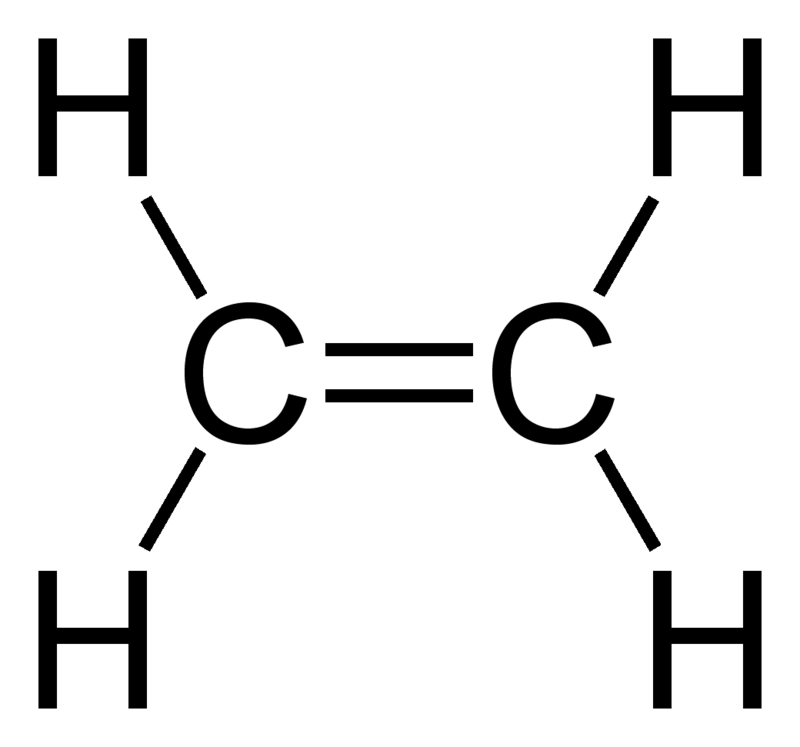
Which Is An Unsaturated Hydrocarbon?
An unsaturated hydrocarbon is a type of hydrocarbon that contains at least one double bond, triple bond, or ring in its carbon chain. This means that the carbon atoms in the hydrocarbon are not fully saturated with hydrogen atoms, as they would be in a saturated hydrocarbon with only single bonds.
Unsaturated hydrocarbons can be classified into two main types: alkenes and alkynes.
1. Alkenes: These are unsaturated hydrocarbons that contain at least one carbon-carbon double bond. The general formula for alkenes is CnH2n, where “n” represents the number of carbon atoms in the chain. For example, ethene (C2H4) and propene (C3H6) are both alkenes.
2. Alkynes: These are unsaturated hydrocarbons that contain at least one carbon-carbon triple bond. The general formula for alkynes is CnH2n-2. For example, ethyne (C2H2) and propyne (C3H4) are alkynes.
Unsaturated hydrocarbons can also form cyclic structures called aromatic hydrocarbons or arenes. These compounds contain a ring of carbon atoms with alternating single and double bonds. The most well-known aromatic hydrocarbon is benzene (C6H6), which has a ring of six carbon atoms with alternating single and double bonds.
Unsaturated hydrocarbons are hydrocarbons that contain double bonds, triple bonds, or rings in their carbon chain. They include alkenes, alkynes, and aromatic hydrocarbons.
Is CnH2n An Unsaturated Hydrocarbon?
CnH2n is indeed an unsaturated hydrocarbon. Unsaturated hydrocarbons are organic compounds that contain carbon and hydrogen atoms and have one or more double or triple bonds between carbon atoms. The general formula for unsaturated hydrocarbons, such as alkenes and alkynes, can be represented as CnH2n for alkenes and CnH2n-2 for alkynes.
Alkenes, which are a type of unsaturated hydrocarbon, have a double bond between two carbon atoms. The general formula for alkenes is CnH2n. This means that for every carbon atom in an alkene molecule, there are two hydrogen atoms.
It is important to note that the general formula CnH2n applies to linear alkenes. When there are branches or rings in the alkene molecule, the formula may vary slightly depending on the specific structure.
To summarize, CnH2n represents the general formula for unsaturated hydrocarbons known as alkenes, which have a double bond between two carbon atoms.
Which Formula Represents An Unsaturated Hydrocarbon C2H6?
An unsaturated hydrocarbon is a type of hydrocarbon that contains at least one double or triple bond between carbon atoms. The molecular formula C2H6 represents the saturated hydrocarbon ethane, which means it contains only single bonds between carbon atoms. Therefore, the formula C2H6 does not represent an unsaturated hydrocarbon.
Conclusion
Unsaturated hydrocarbons are a fascinating group of compounds that contain double or triple bonds in their carbon chains. These compounds have a general formula of CnH2n – 2, indicating that they have fewer hydrogen atoms than the maximum possible for a carbon chain with all single bonds. Alkenes, which are a type of unsaturated hydrocarbon with a double bond between two carbon atoms, have the formula CnH2n.
Unsaturated hydrocarbons are known for their increased chemical reactivity compared to alkanes and aromatic hydrocarbons due to their multiple bonds. These bonds provide opportunities for various types of reactions, making unsaturated hydrocarbons important in fields such as organic chemistry and industry.
Understanding the properties and behavior of unsaturated hydrocarbons is crucial for many applications. For example, alkenes are widely used in the production of plastics, solvents, and synthetic fibers. Additionally, unsaturated hydrocarbons play a significant role in the formation of air pollutants, such as ozone and smog, through reactions with other compounds in the atmosphere.
It is important to note that unsaturated hydrocarbons can exist in different forms, including linear chains, branched chains, and cyclic structures. The presence of double or triple bonds gives these compounds unique properties and makes them valuable building blocks for various chemical processes.
The study of unsaturated hydrocarbons is essential for understanding the intricacies of organic chemistry and its applications in various industries. By further exploring their properties and reactions, scientists can continue to develop new and innovative materials and processes that contribute to technological advancements and a sustainable future.
